Tungsten Trioxide Denitration Catalyst Applies High Temperature Flue Gas Denitration
- Details
- Category: Tungsten Information
- Published on Monday, 13 June 2016 18:31

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
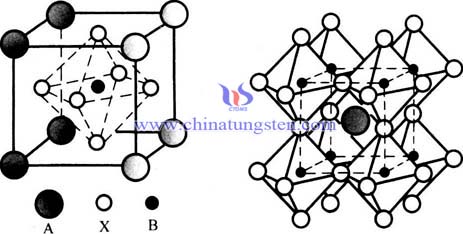
TTB Structures Lead-Free Ferroelectrics
- Details
- Category: Tungsten Information
- Published on Monday, 13 June 2016 18:20

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Maintains Strength of Steel Against Fouling
- Details
- Category: Tungsten Information
- Published on Monday, 13 June 2016 17:40
While we love ceramics and glass, there’s just no denying it—steel is one of the most important materials to modern living. So this is big—researchers at Harvard University’s John A. Paulson School of Engineering and Applied Sciences have devised a way to improve the ubiquitous steel by protecting its surface from fouling and corrosion.
While there are varying grades of steel today, the surface has remained largely unchanged—meaning that steel is still rather susceptible to corrosion and abrasion. Both disrupt the mechanical stability of steel, among other materials, and have a huge economic impact. According to the Wikipedia page on fouling, “one estimate puts the losses due to fouling of heat exchangers in industrialized nations to be about 0.25% of their GDP. Another analysis estimated (for 2006) the economical loss due to boiler and turbine fouling in China utilities at 4.68 billion dollars, which is about 0.169% of the country’s GDP.”
Harvard researchers have developed a scalable technique to give steel a metal oxide coating to prevent liquids from sticking to its susceptible surface. The new coating, a rough nanoporous tungsten oxide layer, “is the most durable anti-fouling and anti-corrosive material to date, capable of repelling any kind of liquid even after sustaining intense structural abuse.
To prevent performance degradation, aka mechanical instability, the team applied the tungsten oxide coating with electrochemical deposition. Instead of creating an even coating, the method grew tiny islands of the metal oxide floating on steel’s surface.
(Accelerated corrosion test, in which unmodified stainless steel (300 grade) (right sample) and the lower part of the TO-SLIPS sample with a 600-nm-thick porous TO film on steel (left sample) were exposed to very corrosive Glyceregia stainless steel etchant. (a–h) Images show corrosion evolution as a function of contact time.)
While that may sound like a weakness for the coating, one of the researchers points out just how valuable those islands are: “If one part of an island is destroyed, the damage doesn’t propagate to other parts of the surface because of the lack of interconnectivity between neighboring islands. This island-like morphology combined with the inherent durability and roughness of the tungsten oxide allows the surface to keep its repellent properties in highly abrasive applications, which was impossible until now.”
The material is tested by scratching it with stainless steel tweezers, screwdrivers, diamond-tipped scribers, and pummeling it with hundreds of thousands of hard, heavy beads,” according to the release. “Then, the team tested its anti-wetting properties with a wide variety of liquids, including water, oil, highly corrosive media, biological fluids containing bacteria and blood. Not only did the material repel all the liquid and show anti-biofouling behavior but the tungsten oxide actually made the steel stronger than steel without the coating.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Bronze Doped Rare Earth Tungsten Electrode Material Preparation
- Details
- Category: Tungsten Information
- Published on Sunday, 12 June 2016 17:45


| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Thin Film Electrode Preparation Method
- Details
- Category: Tungsten Information
- Published on Sunday, 12 June 2016 16:21
Nano semiconductor material used as photocatalyst to photolysis water has gained well efficiency. TiO2 has high catalytic activity and stability is widely used as a kind of photocatalytic material. But its band gap is big (~3.2 eV), it can only be motivated by ultraviolet with short wave length, its light transaction efficiency is low (~4%). Tungsten oxide is an indirect band series transition semiconductor material. Compared to TiO2, it has narrow band gap (2.5~3.0 eV), the relevant absorbing wave length is 410~500nm and well photoelectric responsive property in visible light area.
Tungsten oxide thin film electrode preparation method:
Raw material: FTO glass; tungstic acid; hydrogen peroxide; acetone.
(1) Be ready with clean FTO glass as the substrate of depositing WO3. Cut FTO glass into 1.2cm*2.5cm pieces and clean it by ultrasound and ultraviolet. The clean and flatness of FTO substrate has big effect on adhesive force and uniformity of thin film electrode. So before depositing thin film electrode, the FTO glass should be well cleaned. Firstly, clean the dirties on the surface by ethyl alcohol. Then put the substrate in acetone and ultrasound for 30min to eliminate the ethyl alcohol and oil contamination on the surface. After that, ultrasound it in water for 20 min to eliminate the residual acetone. Finally dry it by nitrogen gas. Then put it into ultraviolet disinfectant tank to sterilize.
(2) Weigh 0.02g tungstic acid and dissolve it by 20ml 30% hydrogen peroxide. Stay it for 12 hours to obtain transparent tungstic acid solution, it will be used as electrolyte solution to deposit WO3.
(3) Use substrate obtained from step (1) as working electrode, measure 30 micro liter tungstic acid solution, dispensing it evenly on the surface of FTO conductive glass. Dry it under room temperature, colorless thin film is obtained.
(4) Put the deposited thin film from step (3) into tube furnace, calcinating it for 2 hours under 500℃, colorless WO3 electrode is obtained.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Photodegradation Organic Pollutant
- Details
- Category: Tungsten Information
- Published on Sunday, 12 June 2016 16:18
Tungsten oxide is an ideal photocatalyst in transition metal oxide which has properties of high catalytic property, low cost, nontoxic and stable. It is now used to degrade organic pollutant such as ethanal, chloroform and fuel into inorganic material. The principle is degrade it into CO2 and H2O, it has high degradation efficient and wide application prospect.
According to thermodynamic argument, the electron hole on the surface of tungsten oxide oxidizes the OH- and hydrone into OH- (free radical). OH- has strong oxidation capability, it can oxidize most of organic and inorganic pollutant and degrade them into innoxious substance like CO2 and H2O. On the other side, active electron on the surface of tungsten oxide has strong reducing capability, it can reduce and remove heave metal ion in the water.
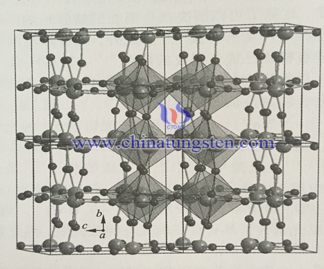
Early research is mainly about applying nano powder semiconductor catalyst in eliminating pollutant in water, but the recovery of catalyst is difficult, it needs dynamic mixing to maintain the suspension of catalyst, the active ingredients loss is significant. Besides that, granule catalyst may cause secondary pollution, it is hard to realize industrialization. In order to overcome the above shortcomings, people use the method of immobilization of photocatalyst which means immobilizing the WO3 catalyst on the glass substrate. However, it lowers the specific surface area of catalyst, causes the reacting area with light reducing, affect catalytic activity. The combination strength of catalyst and substrate reduces, the acid and alkali resistance of substrate material is worse. Thus it isn’t suitable for industrial application.
In recent years, many newly developed nano structure catalyst, such as nanohole, nanotube, nanowire and nanorod. Its large specific surface area can promote the photocatalytic activity and photovoltaic conversion which greatly draw people’s attention. For example, use electrochemical anodic oxidation to prepare WO3 nano porous array can largely enlarge the specific surface area of thin film catalyst. It has better photocatalytic property than powder catalyst.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
The Radiation Shielding Performance Of Tungsten Alloy Shielding
- Details
- Category: Tungsten Information
- Published on Wednesday, 08 June 2016 18:14
 With the continuous development of the radioactive medicine, scientific research and the application of nuclear technology, a variety of radiation rays are widely used in agricultural, industrial, medical, food security and other fields. Radiation is a form of electromagnetic waves and particles (such as α particles, β particles, etc.) diverging from the radiation source to the all directions. According to its level of energy and the ionization ability, it can be classified into ionizing radiation and non-ionizing radiation. Ionizing radiation has sufficient energy to ionize atoms or molecules, while non ionizing radiation cannot. Ionizing radiation mainly includes α, β and γ-rays.
With the continuous development of the radioactive medicine, scientific research and the application of nuclear technology, a variety of radiation rays are widely used in agricultural, industrial, medical, food security and other fields. Radiation is a form of electromagnetic waves and particles (such as α particles, β particles, etc.) diverging from the radiation source to the all directions. According to its level of energy and the ionization ability, it can be classified into ionizing radiation and non-ionizing radiation. Ionizing radiation has sufficient energy to ionize atoms or molecules, while non ionizing radiation cannot. Ionizing radiation mainly includes α, β and γ-rays.
Radiation can cause harm to human body. In the situation of long-term exposure to radiation, the human cells will be destructed or killed on a large scale. Radiation is also one of the causes of childhood leukemia, and can induce the proliferation of human cancer cells, affecting the reproductive system, visual system and cardiovascular system, resulting in mentally challenged children. Therefore, it is necessary to take measures to shield the radiation. The principle of radiation shielding is to set a shielding material between the radiation source and the human body, so that the shielding material interacts with radiation to reduce the number of particles and the energy emitted by the radiation. Generally, radiation shielding materials include non-metallic and organic material, metal and concrete categories, lead shielding and tungsten alloy shielding all belong to metal shielding.
Lead shielding is earliest used for shielding material, but some problems also appeared in the long-term use of lead shielding. Lead and its compounds are toxic to the tissues and organs, its vapor or dust can be inhaled through the respiratory tract or absorbed through the digestive tract into the blood circulation to cause poisoning. While tungsten alloy is nontoxic and does not release toxic substances, neither endanger human health, nor pollute the environment. And compared with lead, tungsten alloy shielding has a better radiation shielding properties that can effectively absorb and shield radiation. Setting a tungsten alloy shielding between the radiation source and the human body can effectively weaken the intensity of the radiation, so that to avoid the radiation damage on the human body.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Cesium Tungsten Bronze Powder and Function Film Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 08 June 2016 17:45
The present invention is preparing cesium tungsten bronze powder through the reactant control, combined with hydrothermal be prepared to effectively simplify the preparation process and reduce the cesium tungsten bronze powder production costs. And in this particular hydrothermal reaction conditions produced cesium tungsten bronze infrared, near-infrared shielding particularly excellent performance.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Picks Failure Mode
- Details
- Category: Tungsten Information
- Published on Wednesday, 08 June 2016 16:16
Tungsten carbide picks are composed of carbide button by the brazing alloy and structural steel, generally used for direct shearer cutting, coal mining, so it is also known as coal buttons. Tungsten carbide picks cut coalseam by impact rotation, which requires withstanding high pressure, shear stress and bending stress at the same time. Not only periodic and alternating impact load, in the process of drilling the friction heat seam cutter and intense friction will be also produced, and throughout the wear process also may be accompanied by local yielding, more contact with the mechanical behavior of fatigue, corrosion and breakage.
Take YG11C tungsten carbide mining button as example, from the fracture of buttons after failure, in the coal excavation process, due to impact load surface at high pressure stress, carbide cutter block collapse occurred. Because coal seam geological uncertainties, buttons can not be held in the excavation and seam completely good contact, and mixed with a certain seam gangue, which also makes poor contact or even inevitable increase the area of contact does not occur. Such a state of stress in the alternating load impact effect is prone to fatigue and thermal fatigue cracking. Furthermore, tungsten carbide pick will be damaged by the stress concentration caused by micro defects on the surface or inside tube.
According to the failure modes and the reasons of tungsten carbide picks, we can improve the properties by adjusting the particle size, the composition and the proportion. First of all, Co content should not be too low, because it can improve the thermal fatigue, enhance plasticity, stress relaxation of the alloy and so on. But increasing Co content will have an influence on the wear resistance to some extent, according to the hardness of the coal seam, generally controlled between 8%-13%. Next, the granularity of WC grains and the proportion is also significant, fine grain WC particles total surface area decreases, the specific surface area increases, the mean free path of Co improves, the equivalent profile improves Co content, will help to improve the fracture toughness of the alloy. In order to enhance Co phase, the use of appropriate heat treatment process is a relatively effective way, and use the existence of polymorphic transition phase to produce other enhanced face-centered cubic cobalt, so as to achieve the purpose of toughening carbide picks.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Shielding Used For Agriculture
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 June 2016 17:57
 With the development of radiation technology, it is widely used in various fields. In the medical field, it is used to diagnose the abnormal changes in tissues and organs of the body and cancer therapy; in industry, it is used in the automated quality control of industrial production line and the detection of the casting crack and weld crack; in the food safety, it is used for food preservation, food sterilization; in agriculture, it is mainly used for radiation breeding, isotopic tracing and radiation disinfestation. Among them, radiation breeding is the use of ionizing radiation to treated with crops to induce mutations, then choosing good variation individuals to cultivate to obtain new varieties; isotopic tracing is the technology that introduce the radioisotope into the body of plants and animals, and then research the growth rhythm or the absorption condition of nutrition of plants and animals by using the radiation detector to track the absorption, accumulation and transfer conditions of isotopes in the body; while the radiation disinfestation is a method that uses radiation source to irradiate injurious insect, resulting in dominant lethal mutations in the body of insects and causing the abnormal mitotic, then overwhelming numbers of sterile insects are released into the wild.
With the development of radiation technology, it is widely used in various fields. In the medical field, it is used to diagnose the abnormal changes in tissues and organs of the body and cancer therapy; in industry, it is used in the automated quality control of industrial production line and the detection of the casting crack and weld crack; in the food safety, it is used for food preservation, food sterilization; in agriculture, it is mainly used for radiation breeding, isotopic tracing and radiation disinfestation. Among them, radiation breeding is the use of ionizing radiation to treated with crops to induce mutations, then choosing good variation individuals to cultivate to obtain new varieties; isotopic tracing is the technology that introduce the radioisotope into the body of plants and animals, and then research the growth rhythm or the absorption condition of nutrition of plants and animals by using the radiation detector to track the absorption, accumulation and transfer conditions of isotopes in the body; while the radiation disinfestation is a method that uses radiation source to irradiate injurious insect, resulting in dominant lethal mutations in the body of insects and causing the abnormal mitotic, then overwhelming numbers of sterile insects are released into the wild.
Tungsten alloy shielding is widely used in the agriculture field due to its excellent radiation shielding performance. Radiation can cause harm to human body by accelerating the decline of the cells, causing cell abnormalities or inhibiting the generation of new cells, or causing changes in the body's biochemical reactions. Long-term exposure to radiation can also lead to serious damage to human organs and systems, as well as causing leukemia, cancer, reproductive system diseases, aplastic anemia and other diseases. Thus, people should pay attention to use the tungsten shielding for the application of radiation technology in agriculture to shield and absorb radiation. Scientists have discovered that the radiation shielding property of a metal material increases with its density, the higher density means the better radiation absorption capacity and radiation shielding ability. Compared to other traditional materials (such as lead), tungsten alloy has higher density, therefore tungsten alloy shielding has better radiation shielding performance. And tungsten alloy shielding is non-toxic and is extremely environmentally friendly shielding material.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com