Tungsten Trioxide Photocatalyst for Inorganic Synthesis
- Details
- Category: Tungsten Information
- Published on Wednesday, 22 June 2016 17:30

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ceramic Coating Tungsten Powder
- Details
- Category: Tungsten Information
- Published on Tuesday, 21 June 2016 17:57
By coating the tungsten powder particles with a ceramic barrier such as alumina, the leachability of tungsten is prevented or greatly reduced. Preferably, the ceramic coating substantially encapsulates each particle of tungsten and has a thickness of at least about 30 nm and, more preferable, from about 200 nm to about 500 nm. Examples of ceramic coatings that may be used include, but are not limited to, aluminum oxide (alumina), aluminum oxyhydroxide (AlOOH), zirconium oxide (zirconia), cerium oxide (ceria), hafnium oxide (hafnia), and magnesium oxide (magnesia). Preferably, the coating is applied in a fluidized bed of tungsten particles using a chemical vapor deposition (CVD) reaction. Similar methods have been used for coating phosphor particles.

The use of tungsten powder or pressed tungsten powder compacts in pure or mixtures with other powders under natural conditions in the presence of water and oxygen (e.g., air or dissolved oxygen) leads to the formation of a water-soluble, tungsten-containing species. The first step of the reaction can be described as follows:
W+H2O+1.5O2-->WO42−+2H+.
The monotungstate ion, WO42−, reacts with H+, resulting in the formation of the soluble metatungstate anion [H2W12O40]6−:
12WO42−+18H+-->[H2W12O40]6−+8H2O.
The formation of this polyoxometalate anion is detectable by its typical UV absorption maximum at 256 nm (molar extinction coefficient, ε256=3.8×104 L(mol·cm)−1).
A spheroidized tungsten powder of (particle size >3 micrometers) was coated with a ceramic material in a fluidized bed reactor using a CVD reaction of trimethylaluminum (TMA) and water vapor. The hydrolyzed TMA coating and CVD process are described in U.S. Pat. The hydrolyzed TMA coating is believed to be an aluminum oxyhydroxide, but may be varied in composition between aluminum oxide and aluminum hydroxide depending upon the reaction conditions. The CVD reaction can be described as follows:
Al(CH3)3+(3+n)/2H2O-->AlO(3−n)/2(OH)n+3CH4(0≦n≦3)
The tungsten particles were coated in the fluidized bed reactor for various lengths of time (from 0.5 hours to 3.5 hours) in order to produce coating thicknesses from 30 nm-500 nm.
Coated and uncoated tungsten powders were subjected to a leach test performed in an aqueous buffer solution. The buffer solution having a pH of 7.2 was prepared by dissolving 4.03 mg KCl, 50.6 mg CaSO4.2H2O, 123.2 mg MgSO4.7H2O, 96.0 mg NaHCO3, and 209.3 mg of a noncomplexing tertiary amine, 3-(N-morpholino) propanesulfonic acid (MOPS) per liter of water. Ten-gram amounts of the powders were added to 500-ml volumes of the aqueous buffer solution in 1-liter Erlenmeyer flasks.
The 1-liter flasks containing the samples were loosely covered with an aluminum foil and continuously shaken in a dark, thermostated room (72° F.) with a LAB-LINE Force orbital open air shaker, Model 4690, for a period of 28 days. Periodic 25-ml samples of the leachate solutions were taken and analyzed for pH, oxygen content, and tungsten content at 7, 14, 21, and 28 days. A constant oxygen concentration of 8.3±0.2 mg/liter was observed for the entire testing period of 28 days.
Coating thickness was determined using a sputtering rate of approximately 0.1 nm/s (based on a tantalum oxide depth-profiling standard). The completeness of the ceramic coating on the particles was evaluated using x-ray photoelectron spectroscopy by observing the attenuation of the tungsten photoelectron peak. In each case, the coating attenuated the W signal by at least 99% indicating that the coating substantially encapsulated the individual tungsten particles.
As can be seen, the amount of tungsten in the leachate of the uncoated spheroidized tungsten powder (uncoated control) increases from 0.43% of the initial tungsten amount at 7 days to 0.75% at 28 days. Whereas, in all cases, the ceramic coating suppressed the leachability of tungsten as compared to the uncoated control and in fact the amount of leached tungsten becomes almost undetectable as coating thickness increases.
| Tungsten Powder Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Denitration Catalyst -- Ultra-Low Emission "Powerful Warrior"
- Details
- Category: Tungsten Information
- Published on Tuesday, 21 June 2016 17:31
 Ultra-low emissions, refers to processes of power generation operation, ending treatment ect. of the thermal power plant coal-fired boiler, a variety of pollutant removal efficiency collaborative integration system technologies are used to make atmospheric emissions of pollutant concentrations in line with emission limits of gas-fired units, i.e. the emission concentrations (reference oxygen content of 6%) of dust, sulfur dioxide and nitrogen oxides are respectively no more than 5 mg / m³, 35 mg / m³, 50 mg / m³, and being the new benchmark of coal-fired generating units of cleaner production level.
Ultra-low emissions, refers to processes of power generation operation, ending treatment ect. of the thermal power plant coal-fired boiler, a variety of pollutant removal efficiency collaborative integration system technologies are used to make atmospheric emissions of pollutant concentrations in line with emission limits of gas-fired units, i.e. the emission concentrations (reference oxygen content of 6%) of dust, sulfur dioxide and nitrogen oxides are respectively no more than 5 mg / m³, 35 mg / m³, 50 mg / m³, and being the new benchmark of coal-fired generating units of cleaner production level.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Photocatalyst Extracts Precious Metal
- Details
- Category: Tungsten Information
- Published on Tuesday, 21 June 2016 17:28
 Precious metal mainly refers to the 8 kinds of metal elements of gold, silver and platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, platinum). Most of these metals have beautiful colors, and quite strong in resistance to chemicals, which means under the normal conditions, chemical reaction is unlikely to be caused. Today, the precious metal is no longer limited to the manufacture of ornaments and play a role in the economic and financial, but widely used in microelectronics, aerospace, missiles, rockets, light industry, petroleum, environmental protection, military and medicine, and many other aspects, much precious metals and their alloys, compounds have shown important technical features in high-tech fields, such as catalysis, electronics, carcinogenic, fine chemicals and so on. Accordingly, the precious metal is also known as vitamin of modern industry.
Precious metal mainly refers to the 8 kinds of metal elements of gold, silver and platinum group metals (ruthenium, rhodium, palladium, osmium, iridium, platinum). Most of these metals have beautiful colors, and quite strong in resistance to chemicals, which means under the normal conditions, chemical reaction is unlikely to be caused. Today, the precious metal is no longer limited to the manufacture of ornaments and play a role in the economic and financial, but widely used in microelectronics, aerospace, missiles, rockets, light industry, petroleum, environmental protection, military and medicine, and many other aspects, much precious metals and their alloys, compounds have shown important technical features in high-tech fields, such as catalysis, electronics, carcinogenic, fine chemicals and so on. Accordingly, the precious metal is also known as vitamin of modern industry.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Heater - Heating Element
- Details
- Category: Tungsten Information
- Published on Tuesday, 21 June 2016 17:03
The heating element is a kind of electronic device which can convert the electrical energy into thermal energy, it is widely used in water heater, dishwasher, oven, toaster, coffee maker, and other devices which need the performance of heat production. The heating element is mainly composed by the electric coil which can be wrapped by insulating material or protective material. When the current pass through the wire, it will generate heat after encountering resistance. The amount of heat can be changed by adjusting electric current. Heating element comprises a short-wave infrared heating tube, medium-wave infrared heating tube, far outside heating pipes and heater band.
 Under normal conditions, the degree of burning of the heating element will increase when time goes by. Therefore, they are often designed to be as accessible style, which can easy to replace a broken heating element. However, replacement of components would cost more. Taking into account this problem, it is better to do a test before replacement to ensure whether the problem is in the heating element. It may because that the electricity had not arrived element, resulting in components cannot generate heat, there may be a problem of wiring, or other reasons.
Under normal conditions, the degree of burning of the heating element will increase when time goes by. Therefore, they are often designed to be as accessible style, which can easy to replace a broken heating element. However, replacement of components would cost more. Taking into account this problem, it is better to do a test before replacement to ensure whether the problem is in the heating element. It may because that the electricity had not arrived element, resulting in components cannot generate heat, there may be a problem of wiring, or other reasons.
The quantity of heat of heating element is mainly due to its metallic material. Tungsten heater is often used as a heating element for vacuum deposition for its good conductive property. The reasons for choosing metallic materials as a part of the heating element are that they are able to withstand repeated heating and cooling, cost less, and have good results with high material utilization. In wet conditions, heat insulation material will spread out the heat, to prevent heating element direct contact with moisture in the air. Most heating elements use Nichrome 80/20 (80% nickel, 20% chromium) wire, ribbon, or strip. Nichrome 80/20 is an ideal material, because it has relatively high resistance and forms an adherent layer of chromium oxide when it is heated for the first time.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
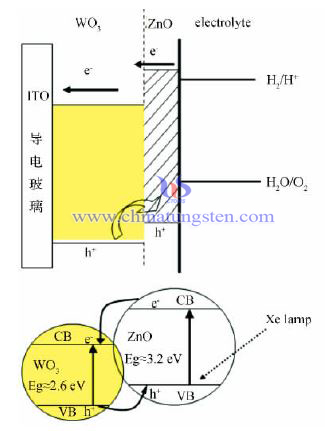
Zn Modified Tungsten Oxide Thin Film Electrode
- Details
- Category: Tungsten Information
- Published on Monday, 20 June 2016 17:43
To promote the photoelectrochemical property, we usually take the following methods:
(1) Loading precious simple metal substance such as Pt, Ag, Au. Lay WO3 over FTO with Ag grid, the testing photocurrent density is two times than the one without Ag.
(2) Doping certain amount of metal ion or non-metal ion in WO3. Doping Ta5+ in WO3 photo electrode, the experiment shows that photoelectric conversion rate of doping electrode is much higher.
(3) Recombine WO3 with other inorganic semiconductor material, use impregnation method to prepare CuO/WO3 composite material. It turns out that CuO/WO3 shows better photo catalytic ethylal.
(4) Recombine WO3 with organic material. Prepare PBrT/WO3 and PMOT/WO3 composite material. After testing it shows that the composite one has better electrochemical property.
This paper mainly focuses on the research of Zn affects WO3 thin film photoelectrode photoelectrochemical property. Use simple cathode electro deposition-impregnation method heating for 3hours in 450℃ to prepare Zn modified tungsten oxide thin film electrode. By testing the photoelectrochemical and photoelectric catalytic activity, a certain amount of Zn doping WO3 photoelectrode, its property is greatly improved.
Preparation method:
(1) Use Pt electrode as counter electrode, the saturated calomel electrode as reference electrode, cleaned indium tin oxide electric glass as working electrode. The electro deposition is carried out under room temperature, the applied voltage is -0.6V, deposition time is 1 hour, then blue amorphous form of WO3-x·nH2O thin film is obtained.
(2) After dry in the air, put the thin film in muffle furnace, annealing for 3 hours under 450℃, the heating rate is 2℃·min-1, then we can get tungsten oxide thin film electrode.
(3) Use electro deposition-impregnation method to modify WO3 electrode with Zn. Impregnate thin film in Zn(NO3)2 solution. After dry it in the air, annealing the WO3 thin film contained with Zn in muffle furnace under the same condition with step(2). Finally we can get Zn modified tungsten oxide thin film electrode.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide in Polymer Electrolyte Fuel Cell Electrode
- Details
- Category: Tungsten Information
- Published on Monday, 20 June 2016 17:41
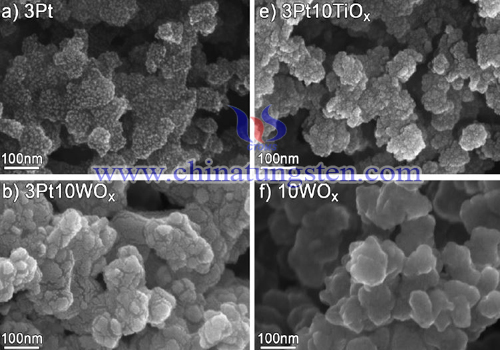
Development of new alternative electrode materials is essential in order for the polymer electrolyte fuel cell (PEFC) to be able to reach a broad market. Today, high platinum loadings are required, especially on the cathode, to obtain sufficient activity for oxygen reduction. In addition, electrode degradation causes loss of catalyst surface area and requires high initial loadings to maintain the cell performance over time. There are problems related to Pt also on the anode side where poisoning of the catalyst, by e.g. CO, reduces the activity.
Approaches to improve the electrodes and reduce their costs are continuously evaluated and include alternative catalysts or supports as well as new structures and morphologies of the catalyst layer. Alternative catalysts, based on non-precious metals, Pt alloys/mixtures, and/or novel supports should preferably reduce the total amount of Pt, increase the activity, and be stable in the fuel cell environment. The support material can influence the activity by spill-over effects as well as changing the electronic structure of the catalyst. New support materials can improve the activity, utilization, and stability of the catalyst or of the support itself.
Tungsten oxide is a material which has been extensively investigated for a wide range of applications, mainly, due to its unique electrochromic properties but also for its electrocatalytic activities. The electrochromism allows tungsten oxide to intercalate/deintercalate ions (of e.g. H, Li, Na, K, Pb, Cd) into its structure in the formation of tungsten bronzes. The most widely studied form is the hydrogen tungsten bronze where protons are inserted in the oxide structure as HxWO3 and 0 < x < 1. The bronze formation mechanism has been the subject for numerous studies and it is suggested that the hydrogen atoms form hydroxyl bonds in the tungsten oxide.
The bronze formation is greatly affected by the water content, porosity, and also crystallinity, which in turn affect the catalytic properties of tungsten oxide. At the same time as protons can be incorporated in the WOx structure, they also have a significant mobility which means that WOx functions as a proton conductor under these conditions. Since the hydrogen tungsten bronze formation is dependent on the water content, large variation in conductivity has been reported when varying the relative humidity. Moreover, Pt supported on tungsten oxide has been shown to affect the bronze formation and both an increased intensity of the hydrogen intercalation/deintercalation peaks as well as a shift of the peak potential to higher potentials has been reported.
Tungsten oxide has been evaluated both as support and active catalyst in fuel cell anode as well as cathode electrodes. Sole tungsten oxide has displayed activity for hydrogen oxidation, which was attributed to high porosity and high surface area. Combined Pt and tungsten oxide based catalysts have been investigated for methanol/ethanol oxidation, CO oxidation, hydrogen oxidation as well as oxygen reduction. For methanol oxidation, the Pt on WOx system has shown improved efficiency over Pt catalyst due to both the spill-over of hydrogen from Pt to WOx but also the ability of WOx to provide oxygen atoms at low potentials and thereby avoiding CO-poisoning. Others have attributed the improved performance to an increased electrochemical active surface area (ECSA) of Pt on WOx.
Tungsten oxide is also relatively stable in acidic environment, which is a prerequisite for use in polymer electrolyte fuel cell applications. However, some dissolution of tungsten oxides has been reported. In a previous study, we examined the impact of different metal oxides on the stability and activity of platinum in thin model cathodes in a PEFC. Pt on WOx did exhibit an improved activity for oxygen reduction and possibly also an improved stability compared to Pt alone. Interesting features such as reduced platinum oxide formation and platinum catalyzed hydrogen tungsten bronze formation were also seen when Pt was deposited on WOx.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide in Polymer Electrolyte Fuel Cell Electrode
- Details
- Category: Tungsten Information
- Published on Monday, 20 June 2016 17:41
Development of new alternative electrode materials is essential in order for the polymer electrolyte fuel cell (PEFC) to be able to reach a broad market. Today, high platinum loadings are required, especially on the cathode, to obtain sufficient activity for oxygen reduction. In addition, electrode degradation causes loss of catalyst surface area and requires high initial loadings to maintain the cell performance over time. There are problems related to Pt also on the anode side where poisoning of the catalyst, by e.g. CO, reduces the activity.
Approaches to improve the electrodes and reduce their costs are continuously evaluated and include alternative catalysts or supports as well as new structures and morphologies of the catalyst layer. Alternative catalysts, based on non-precious metals, Pt alloys/mixtures, and/or novel supports should preferably reduce the total amount of Pt, increase the activity, and be stable in the fuel cell environment. The support material can influence the activity by spill-over effects as well as changing the electronic structure of the catalyst. New support materials can improve the activity, utilization, and stability of the catalyst or of the support itself.
Tungsten oxide is a material which has been extensively investigated for a wide range of applications, mainly, due to its unique electrochromic properties but also for its electrocatalytic activities. The electrochromism allows tungsten oxide to intercalate/deintercalate ions (of e.g. H, Li, Na, K, Pb, Cd) into its structure in the formation of tungsten bronzes. The most widely studied form is the hydrogen tungsten bronze where protons are inserted in the oxide structure as HxWO3 and 0 < x < 1. The bronze formation mechanism has been the subject for numerous studies and it is suggested that the hydrogen atoms form hydroxyl bonds in the tungsten oxide.
The bronze formation is greatly affected by the water content, porosity, and also crystallinity, which in turn affect the catalytic properties of tungsten oxide. At the same time as protons can be incorporated in the WOx structure, they also have a significant mobility which means that WOx functions as a proton conductor under these conditions. Since the hydrogen tungsten bronze formation is dependent on the water content, large variation in conductivity has been reported when varying the relative humidity. Moreover, Pt supported on tungsten oxide has been shown to affect the bronze formation and both an increased intensity of the hydrogen intercalation/deintercalation peaks as well as a shift of the peak potential to higher potentials has been reported.
Tungsten oxide has been evaluated both as support and active catalyst in fuel cell anode as well as cathode electrodes. Sole tungsten oxide has displayed activity for hydrogen oxidation, which was attributed to high porosity and high surface area. Combined Pt and tungsten oxide based catalysts have been investigated for methanol/ethanol oxidation, CO oxidation, hydrogen oxidation as well as oxygen reduction. For methanol oxidation, the Pt on WOx system has shown improved efficiency over Pt catalyst due to both the spill-over of hydrogen from Pt to WOx but also the ability of WOx to provide oxygen atoms at low potentials and thereby avoiding CO-poisoning. Others have attributed the improved performance to an increased electrochemical active surface area (ECSA) of Pt on WOx.
Tungsten oxide is also relatively stable in acidic environment, which is a prerequisite for use in polymer electrolyte fuel cell applications. However, some dissolution of tungsten oxides has been reported. In a previous study, we examined the impact of different metal oxides on the stability and activity of platinum in thin model cathodes in a PEFC. Pt on WOx did exhibit an improved activity for oxygen reduction and possibly also an improved stability compared to Pt alone. Interesting features such as reduced platinum oxide formation and platinum catalyzed hydrogen tungsten bronze formation were also seen when Pt was deposited on WOx.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Hollow Ball Tungsten Trioxide Photocatalyst
- Details
- Category: Tungsten Information
- Published on Monday, 20 June 2016 17:37
 Tungsten trioxide has been researched a lot, because of its unique physical and chemical properties, such as electrochromic, gas properties, photoluminescence. In addition, the tungsten oxide has non-toxic, and narrow band gap (2.4eV ~ 2.8eV), as a semiconductor material used in the visible light photocatalyst catalytic reactions of organic matter in sewage catalytic degradation. Hollow structural material has both inner and outer surfaces, and can take advantage of multiple reflections of light into the interior, which has a unique application.
Tungsten trioxide has been researched a lot, because of its unique physical and chemical properties, such as electrochromic, gas properties, photoluminescence. In addition, the tungsten oxide has non-toxic, and narrow band gap (2.4eV ~ 2.8eV), as a semiconductor material used in the visible light photocatalyst catalytic reactions of organic matter in sewage catalytic degradation. Hollow structural material has both inner and outer surfaces, and can take advantage of multiple reflections of light into the interior, which has a unique application.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Powder Classification Application in Coarse Tungsten Carbide Powder Production
- Details
- Category: Tungsten Information
- Published on Monday, 20 June 2016 17:34

| Tungsten Powder Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com