WO3 Nano Material Photocatalytically Splitting of Water into Hydrogen
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 June 2016 17:56
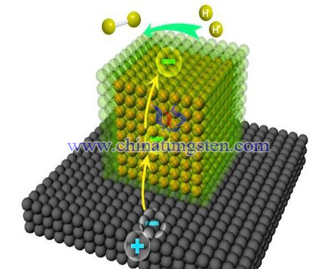
WO3 nano material has the advantages of innocuous and unpoisonous, easy to produce, stable property, cost efficient, visible light response. It is a kind of ideal photoanode semiconductor material in photoelectrochemistry reaction and is widely used in photoelectrochemistry field (photocatalytic water splitting, photocatalytic organic pollutant and solar cell.
In 1972, Fujishima and Honda firstly reported that under lighting conditions, using photoelectrochemistry cell composed by TiO2 semiconductor electrode to decompose water into hydrogen and oxygen. From then on many scientific researchers did a lot of study on other oxide semiconductors to photocatalytically splitting of water into hydrogen.
Decompose 1mol H2O into hydrogen and oxygen need 273kJ power under normal condition, which equals to 2.46eV. Voltage needed for common electrolysis of water reaction is 1.23V. So if using semiconductor to phorocatalytically decompose water, the theoretical band gap of material should be higher than 1.23eV. In actual reaction, the overpotential and electrode polarization may cause losing power, so the band gap should be 2.0~2.2eV.
According difference in structure of material, the band gap of WO3 is 2.5~2.8eV, it is an ideal photocatalytically splitting water material. Research shows that when pH=0, electrode potential at the bottom of conduction band of WO3 is +0.4V which is higher than regular value, so it can not be used in oxygen evolution reaction. However, due to the strong oxidizability of valance, it can be used to produce oxygen by decomposing water.
In 1976, scientist Hobes from Israel firstly used WO3 in photocatalytically splitting of water into hydrogen. Compared to TiO2 photocatalyst, light conversion rate of WO3 is lower. But WO3 has advantages like innocuous and unpoisonous, easy to produce, stable property, cost efficient, visible light response. Besides that, in actual photocatalytically splitting water, WO3 can maintain favorable anti light corrosive property and optical electronic transmission under strong sun light. Thus it is a kind of ideal photocatalyst for splitting water.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Plutonium-239 Tungsten Radiation Shielding
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 June 2016 17:54
 Plutonium-239 is a fissile isotope of plutonium with a half-life of 24,110 years, which is mainly used for the production of fissile material or other materials, or used for industrial-scale irradiation. Due to plutonium-239 can produce large amounts of pure plutonium-239 at a lower cost than the high enriched uranium -235, it is also widely used in nuclear weapons and nuclear power plants. Plutonium-239 is artificially produced by fast neutrons in reactor to bombard uranium - 238, a fissile plutonium-239 atom can produce 207.1 MeV energy. In the reactor, the uranium-235 atoms yield two or three neutrons which would be absorbed by uranium – 238 to produce plutonium-239 and other isotopes. In fact, the role of production reactor is to burn part of the uranium-235 in exchange for plutonium-239, and per burn of a uranium-235 atom will get eight plutonium-239 atoms. Plutonium-239 can also absorb neutrons and has the least critical mass in all commonly used nuclear fuel.
Plutonium-239 is a fissile isotope of plutonium with a half-life of 24,110 years, which is mainly used for the production of fissile material or other materials, or used for industrial-scale irradiation. Due to plutonium-239 can produce large amounts of pure plutonium-239 at a lower cost than the high enriched uranium -235, it is also widely used in nuclear weapons and nuclear power plants. Plutonium-239 is artificially produced by fast neutrons in reactor to bombard uranium - 238, a fissile plutonium-239 atom can produce 207.1 MeV energy. In the reactor, the uranium-235 atoms yield two or three neutrons which would be absorbed by uranium – 238 to produce plutonium-239 and other isotopes. In fact, the role of production reactor is to burn part of the uranium-235 in exchange for plutonium-239, and per burn of a uranium-235 atom will get eight plutonium-239 atoms. Plutonium-239 can also absorb neutrons and has the least critical mass in all commonly used nuclear fuel.
Plutonium-239 is toxic, and can emit α particle. α particle is mainly consist of two protons and two neutrons, which is equivalent to the kernel of helium 4 or helium 4 after ionization(i.e., He2 +). Generally the chemical element which is radioactive and has a larger atomic weight can emit α particle through α decay to convert into lighter elements, until the element is stable. Since the volume of α particle is larger and it has two positive charges, it can easily ionize other substances. Once the radioactive materials that can emit α particles (such as radiation fog) are inhaled or eating by human, α particle can damage organ cells directly. Although the penetrating power of α particle is weak, its strong ionization ability to cause harm to biological organism is no less than that of other radiation.
Plutonium-239 tungsten radiation shielding is used to shield the radiation produced by the radioactive isotope plutonium-239. Compared with the conventional shielding materials (such as lead), tungsten alloy shielding reflects good radiation shielding effect. Lead is early used in the shielding field, but a discovery shows that the radiation shielding performance of lead shielding is not high enough and it can also seriously pollute the environment. Under the condition of the same thickness, the radiation shielding capability of the tungsten shielding is twice as good as lead shielding.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Doped Tungsten Bronze Powder Synthetic Method
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 June 2016 17:46

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Powder Granularity Effect on Armor Capability of Tungsten Copper Liner
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 June 2016 17:16
In recent years, tungsten copper is one of the most outstanding materials for the liner. Liner is the key part of shaped charge, which quality directly determines the penetration and armor capability of warhead blast. Compared with conventional liner, the powder liner has many advantages, such as high density, excellent thermal conductivity, good elongation, flexible composition ratio, better moldability and so on. Penetrating analysis based on fluid dynamics theory, it can be avoided to a large extent from the phenomenon of blocking pestle to form a longer and stable jet. Tungsten powder shape, size and size distribution can have a significant impact on the powder compaction and sintering process and the final product performance, thus affecting the performance liner shaped and penetration depth.
The experiment uses mixed - pressing sintering process, and put the compacting liner blank into high-temperature tube furnace, after 70min gradually warmed to 750℃ and holding 20min. Then use instantaneous electric detonator at the top of the midpoint detonating the static penetration power experiment. From the uniformity of powder liners isometric high radial position analyses, its runout and wall thickness substantially unchanged. The density distribution is analyzed in the axial direction, typically the maximum density at the top of the bottom of the minimum density, a decreasing trend was up and down. Detecting the use of Archimedes powder liners green density can be found in between 45-62μm tungsten powder particle granularity, with the decrease of tungsten powder particle size, density and relative density of the compact tungsten-copper powder liner and sintered density showed a trend of increasing. While maintaining the same quality with sub-fractions, process, particle size and other conditions of 60 ° tungsten powder copper shaped the static penetration test can be found with the decrease of the particle size of tungsten powder, powder liner break a depth showed an increasing trend and a significant improvement in the performance of armor.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Manufacturing Process of Tungsten Heater
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 June 2016 10:34
Tungsten heater may also be known as tungsten evaporator coil, which is made by special heat treatment process, and it has a good corrosion resistance and excellent thermostability. It can be used for plastic color plating, optical fiber deposition coating gold or aircraft industry, film, photography, and scientific research. The heart of the vacuum coating process is a tungsten heater as a heating source.
Wolfram heater is generally made of high-temperature multi-strand tungsten wires twisting, hot - forming, cleaning, surface treatment, heat-setting and other processes. Every procedure has to be controlled in strictly technology requirements. The twisting process is to use more than two tungsten wire are interwoven together in accordance with a predetermined direction, in order to ensure that there is a certain cross-section on the electrical properties and a certain degree of flexibility of the mechanical properties. Then use special equipment for the molding pressing. Next is to the cleaning and surface treatment of the heater, making its surface has no visible cracks and the contaminants, such as oxides and drawing lubricants. Contaminants on surface will have an impact in the vacuum content, and play a negative role on the follow-up free metal evaporation rates. The final step is to heat setting process. It is necessary for this process to strictly control the recrystallization temperature of tungsten. Heat setting refers to the use of heat, eliminate the internal stress generated in the fabric fiber drawing process, so that a certain degree of relaxation macromolecules so fixed woven fibers forming the shape. This process is to use heat to eliminate the internal stresses formed by stretching process, slacking macromolecule and finalizing the design of weave fiber. This process can improve the stability of the size of wolfram heater and reduce washing shrinkage. What’s more, it can eliminate wrinkles and improve its crease resistance and the comprehensive performance. When heat setting for wolfram heater, it should not be processing in a high temperature, avoiding surface oxidation.
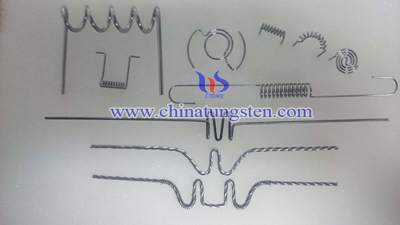
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Hexagonal Tungsten Bronze Rod Nano Particles and Preparation
- Details
- Category: Tungsten Information
- Published on Monday, 06 June 2016 17:47
1. Through exchange resin - sol-gel method or rapid acidification Preparation of solid colloidal tungstic acid; wherein: exchange resin sol - gel method comprises the following steps: (1) tungstate dissolved in water to obtain a concentration of tungstate solution 0.1~2mol / L of; (2) the use of cation exchange resin tungstate solution into acid solution; (3) the acid solution was allowed to stand in 0~100 ° C under aging 0.01~48h make gel to give a gum solid acid. The rapid acidification method comprises the following steps: (1) tungstate dissolved in water to obtain a concentration 0.01~5mo 1 / L of the tungstate solution; (2) under agitation excess acid solution was quickly added to the tungstate solution to PH <1; said acidic solution is a lactic acid, tartaric acid, acetic acid, oxalic acid, hydrochloric acid, sulfuric acid, nitric acid, citric acid or a mixed solution, the concentration 0.1~18.4mol / L; (3) continuously subjected to suction filtration after stirring colloidal floc formation resulting solid suspension tungstate gum acid. Tungstates described as: sodium tungstate, potassium tungstate, lithium tungstate, cesium, calcium tungstate, tungsten, bismuth, tungsten acid, silver, tungsten, zinc, magnesium and tungsten, partial ammonium tungstate, ammonium tungstate positive, ammonium paratungstate, alkali metal tungstate one or a mixture thereof.
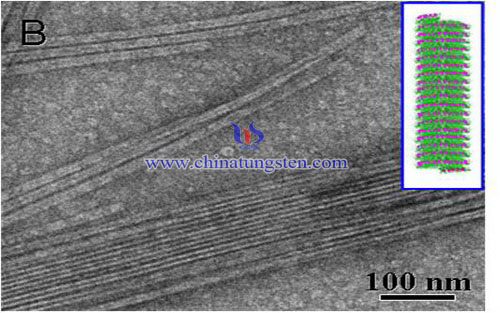
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Nanowire
- Details
- Category: Tungsten Information
- Published on Monday, 06 June 2016 17:38
In recent years, one-dimensional nano material such as nanowire, nanorod, nanotube are becoming popular due to its special properties. Tungsten oxide is a special N type semiconductor material, and also one of the oxide semiconductors which can realize quantum size effect. It shows great property in electrochromism, photochromism, gasochromism and is applied in various fields like chemical sensor, fuel cell and electronic device.
Tungsten oxide has crystalline form of orthogonality, monoclinic, cube and hexagonal. Among which hexagonal tungsten oxide is paid much attention due to its special hexagonal panel, many metal ion can inlaying in it. So as to form hexagonal tungsten bronze MxWO3( M = Li +、Na +、K +). It shows great application prospect in negative electrode materials and Iithium ion rechargeable battery.
Use sodium tungstate and hydrochloric acid as raw material, potassium oxalate and potassium sulphate as additive, hexagonal tungsten oxide nanowire can be synthesized by hydrothermal method.
Raw material: Na2WO4·2H2O; hydrochloric acid, potassium oxalate; potassium sulphate; absolute ethyl alcohol, all analytic grade; deionized water.
Preparing method:
(1) Weigh 3.68g Na2WO4·2H2O to dissolve in 20ml deionized water, stir it and adding 3mol HCL until PH=1.
(2) Keep stirring until there is no light yellow anticipation any more, adding in certain amount of K2C2O4 and K2SO4, keep stirring for 30min.
(3) Move the solution to 100ml stainless ste with polytetrafluoroethylene liner, adding deionized water to 3/4 of the reactor, seal and react under 150℃ for 12h.
(4) Cool it down to room temperature, wash it by deionized water and absolute ethyl alcohol, dry under 60℃, tungsten oxide nanowire is obtained.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Nanotube
- Details
- Category: Tungsten Information
- Published on Monday, 06 June 2016 17:35
When particle size of some material reaches to nano grade (1~100nm), it can show many special reactions. It can be widely applied in functional information display, catalyst, magnetic material. Among which, tungsten oxide has various crystalline structure, it has a large amount of non-stoichiometry oxide form and has multi-functional broad-band gap semiconductor material. WO3 is widely applied in gas sensor, photocatalyst, gasochromism, electrochromism, photochromism and solar cell due to its unique electricity, optical configuation, and magnetic property. Apart from that, temperature induces structure phase transition which results in the changes of volume, resistance and color greatly draw people’s attention. The research on its special structure has become the focus of recent study.

The traditional preparing method of tungsten oxide nanotube need extra coating and sculpture process, the quality of nanotube greatly depends on the control of processing steps. By coaxial electrospinning, hollow nano fiber can be obtained, manufacturing process is simplified.
Raw material: PVA; ammonium metatungstate; absolute ethyl alcohol; aluminum foil; deionized water.
Preparing process:
(1) Weigh a certain amount of PVA to dissolve in deionized water, stirring it for 4hous in constant temperature of 80℃ and prepare it to 15% PVA solution. Then add 24ml alcohol and 6g ammonium metatungstate solution into 60ml PVA solution.
(2) Dilute another 15% PVA solution to 10% as inner pipe solution by deionized water. Inject both inner and outer pipe solution into injector, then inject them into electrostatic spinning coaxial needle.
(3) Under electric voltage 9~15kV, keep spinning with receiving distance of aluminum foil for 10~12cm. Dry the obtained composite fiber in thermotank for 12 hours, keep the temperature to 600℃ by putting it in muffle furnace, and then cool it down to get WO3 nanotube.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Shielding Used For Sterile Insect Technique
- Details
- Category: Tungsten Information
- Published on Monday, 06 June 2016 17:17
 The sterile insect technique is a biological insect control method that uses radiation source to irradiate injurious insect, resulting in dominant lethal mutations in the body of insects (ie, chromosome breakage) and causing the abnormal mitotic, then overwhelming numbers of sterile insects are released into the wild. The released insects are normally male, as the females cause the damage usually by laying eggs in the crop. The sterile males compete with wild males to mate with the females. Females that mate with a sterile male produce no offspring, thus reducing the next generation's population. Repeated release of sterile males can diminish small populations, although success with dense target populations has not been demonstrated. The sterile insect technique has the advantage of strong specificity, and can avoid the resistance to drugs generated by the application of pesticides to prevent insects.
The sterile insect technique is a biological insect control method that uses radiation source to irradiate injurious insect, resulting in dominant lethal mutations in the body of insects (ie, chromosome breakage) and causing the abnormal mitotic, then overwhelming numbers of sterile insects are released into the wild. The released insects are normally male, as the females cause the damage usually by laying eggs in the crop. The sterile males compete with wild males to mate with the females. Females that mate with a sterile male produce no offspring, thus reducing the next generation's population. Repeated release of sterile males can diminish small populations, although success with dense target populations has not been demonstrated. The sterile insect technique has the advantage of strong specificity, and can avoid the resistance to drugs generated by the application of pesticides to prevent insects.
The radiation sources used by sterile insect technique are mainly α-rays, β-rays, γ-rays and neutrons, these rays all belong to ionizing radiation that has ionization effect and will cause some harm to human body. When the body is subjected to the radiation exposure, dizziness, fatigue, headache, memory loss, vision loss, sleep disorders and other symptoms will appear, and the radiation will affect the human reproductive system, circulatory system, immune system, metabolic system, cardiovascular system, resulting in children's mental defects, cancer, leukemia and other diseases. Therefore, people should pay attention to use radiation shielding to shield radiation in the use of inherited sterility technique for the prevention of pests.
Tungsten alloy shielding can be used for shielding the radiation generated in the use of inherited sterility technique. Studies have shown that the radiation shielding capability of a material is closely related to its density, the higher density means a better radiation shielding capability and radiation absorbing ability. Compared to other radiation shielding materials (such as lead), tungsten alloy has a higher density, so that tungsten alloy shielding has better radiation shielding capability.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Nanorod
- Details
- Category: Tungsten Information
- Published on Monday, 06 June 2016 17:16
Since indium storage property of nanocrystalline transition metal oxide (MO,M=Co, Ni, Cu, Fe) is found, other transition metal oxide such as CuO, Fe2O3, Fe2O4, Co3O4, WO3 can be transited by chemical reaction MOx+2xLi+=M+xLi2O. Its capacity is far better than graphite anode material in lithium ion battery. Among which WO3 is the most stable oxide of tungsten under room temperature. It is not only environmental friendly, but also is cheap. It has potential to be widely applied as lithium ion battery anode material. However, WO3 in lumpish has low electric conductivity, its volume changes greatly during charge-discharge process. Thus results in the instability of WO3. One of the improving methods is to synthesis WO3 nano material of different appearance. Then lithium storage property of the material is improved.

Preparing method: By hydrothermal method, tungsten oxide nanorod can be prepared on the indium tin oxide substrate.
Raw material: Sodium tungstate (AR: analytically pure); NaCl (AR); oxalic acid (AR); methylene blue (AR); hydrochroric acid. All solution is prepared by deionized water. ITO electric glass is under ultrasound for 10min in acetone, ethyl alcohol and deionized water, then dry it.
Preparing process:
(1) Dissolve 8.25g sodium tungstate in 25ml deionized water, then adding hydrochloric acid to adjust PH value into 2.0.
(2) Then dilute the solution to 250ml, put in PH meter, adding oxalic acid into solution, adjust PH value to 2.3, the precursor solution is obtained.
(3) Adding 0.3g sodium chloride into hydrothermal reactor, put in the ITO glass and make sure it is slanted. Then adding 20ml precursor solution, sealed it and thermal reacting for 4 hours under 170℃.
(4) After the reaction is done, cool it down to room temperature. Clean the ITO with deionized water and dry it. Tungsten nanorod of even size and bigger density is obtained.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |




 sales@chinatungsten.com
sales@chinatungsten.com