Cesium Tungsten Bronze Ultrafine Powder Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 15 June 2016 18:05


| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Thin Film Electrode Cyclic Voltammetry
- Details
- Category: Tungsten Information
- Published on Wednesday, 15 June 2016 17:27
To study the cyclic voltammetry of tungsten oxide thin film electrode, use three-electrode system, sulfuric acid solucion as electrolyte, the property is observed by measuring light current. Below is cyclic voltammograms of WO3 thin film electrode being heat treatment under 450℃ under dark and 500W xenon light source (light strength 100Mw/cm2). We can see that under darkness the polarization current of electrode is small within the scanning range, it is far smaller than anode polarization current under light. Photoelectrochemical reaction under light has good reversibility. Within electric potential 0.35~1.2V(vs.Ag/AgCl), cathode Pt and anode WO3 thin film electrode reaction is as following:
Anode:2OH-+ h+ → O2 ↑+ 2H+
Cathode:2H+ + 2e- → H2 ↑

When exposed to light, if the applied bias is low, the Fermi level of WO3 is higher, electrolysis solution accepter is easier to trap photo electron near WO3 electrolyte interface of electrode, so the photo current of anode is weaker, even comes close to 0. With the increasing of bias, Fermi level of WO3 decreases as well, the accepter of electrolyte to trap the photo electron is getting harder which makes the photo electron largely spread to the electric substrate. When bias comes to a certain level, the extra electric filed enlarges the migration rate of photo electron, so photo current of anode strengthens with the shuffling of current.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nanoporous Tungsten Oxide Electrode
- Details
- Category: Tungsten Information
- Published on Wednesday, 15 June 2016 17:25
Electrochemical oxidation preparing nanoporous tungsten oxide electrode:
1) Treating method for tungsten foil: Firstly cut it into 10mm x 15mm pieces, using waterproof abrasive to polish it, then clean it with acetone, isopropanol, methyl alcohol and deionized water ultrasound cleaning for 15min, blow it with nitrogen gas.
2) Use tungsten foil as anode, Pt foil of 10 x 15mm as counter electrode, put them into electrobath, the distance between two electrodes is 25mm. Then put electrobath in water bath of constant temperature, adjust the bath temperature to control the reaction temperature. The reacting area is 0.88cm2. Adding a certain amount of ready-prepared 1mol/L(NH4)2SO4 solution electrolyte with different concentration of NH4F.
3) Clean the ready-prepared WO3 nanoporous thin film with deionized water, blow-dry with nitrogen gas in the air and put them in muffle furnace, the heating rate is 5℃/min, cool it down to room temperature, then packed it to nanoporous tungsten oxide electrode by epoxy.
Electrochemical property:
1) Quantum conversion rate
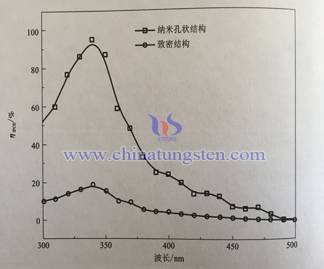
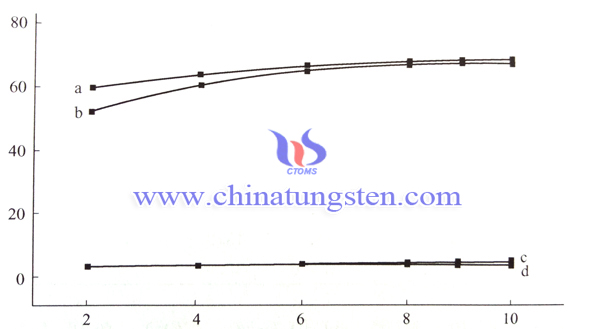
Below is WO3 electrode of nanoporous structure and densification photoaction spectra. Electrolyte solution uses H2SO4 solution of 0.5mol/L(pH=0), electrode potential (vs.Ag/AgCl)is 1.2V, from the spectra we can see nanoporous electrode photoelectron conversion rate is 89.5% within 340nm of ultraviolet area, The conversion rate can reach 22.1% in the visible light area of 400nm. On the contraty, densification structure WO3 electrode conversion rate is only 19.2% and 2.4%, it is far away from nanoporous electrode conversion.
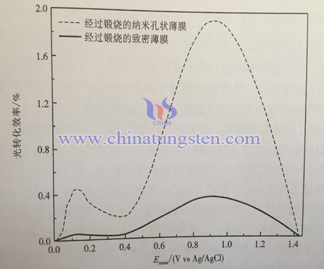
2) Photocurrent density spectra and photoconversion efficiency
Current density of semiconductor photo-anode reflects photocatalytic activity of electrode material. The photocurrent spectra of two different structure electrode is as following. In the dark, the current density of samples are weak; when electrode is exposed to light and with the increase of bias, the photocurrent density also increases. It means that nanoporous WO3 electrode has larger specific surface area, has stronger light absorption ability, it can get full contact with electrolyte and is easier to let photoelectron transport, so it has fine photoelectric property.
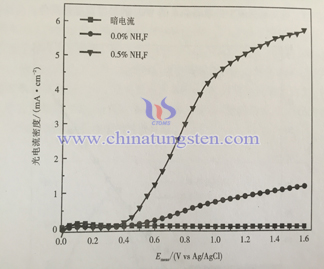
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Throat Insert Used For Rocket
- Details
- Category: Tungsten Information
- Published on Wednesday, 15 June 2016 16:04
Tungsten copper throat insert fabricated by the infiltration process, which not only has high hardness, high density, high strength, high melting point, low coefficient of thermal expansion and excellent wear and corrosion resistance of W, but also has excellent ductility, thermal and electrical conductivity of Cu. What’s more, since there is a great difference in the melting point between W and Cu, at high temperature higher than the melting point of Cu, Cu will evaporate and take away the most of heat and the hard phase W left. So it ensures the stability of throat insert works, and tungsten copper is also known as sweating heat sink material.
Through related experiments the researchers found that coarse grain has better performance in thermal shock resistance and poor in ablation resistance, conversely. Rocket spray tube entrained within particles or liquid phase stream high burning rate will continue to scour throat insert, and this two-phase flow weakly oxidizing, such throat insert constantly ablated, which is a complex physical and chemical process covered by heat conduction, mass conduction, transmission capacity and chemical reactions. According to different ablation principles, it can be specifically divided into thermal ablation, melted ablation and mechanical erosion. When the surface temperature of tungsten copper throat insert is higher, melted copper was deposited on the inner surface, which will cause thermal effect and prevent heat transfer to the inside of the material.
In addition, when the gas temperature below the melting point of tungsten, tungsten skeleton does not melt, ablation occurs at this time of the melting of copper and tungsten substrate by gas particles (such as Al2O3) erosion produced by mechanical erosion. Currently, copper tungsten throat insert prepared have been able to successfully applied to 3600 ℃, 6.88MPa under thermal environment at this temperature above the melting point of W, tungsten skeleton is also possible to melt ablation. From the view point of throat insert structure, improving the roughness and prolonging the length of the straight section can remarkably improve the ablation resistance of throat insert.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Throat Insert
- Details
- Category: Tungsten Information
- Published on Wednesday, 15 June 2016 16:02
Solid rocket motor spray tube by controlling the expansion of the combustion chamber exhaust gas generated energy is efficiently converted to kinetic energy, so as to provide the required power for the aircraft. Throat insert located in the larynx of the spray tube, which plays an important role in restricting the throat area expansion by ablations and the decreases of the thrust. Throat insert usually need to be heated from room temperature to more than 2000℃ and produces a great temperature gradient and thermal stress when the engine working, which is also the main reason for the cracks and failure of throat insert materials. Furthermore, such high-performance engine for rocket often uses metal powders (such as Al) as propellants combustion at high temperature of about 3000 ℃ high burning rate entrained flow of solid particles or liquid droplets (Al2O3) and scoured the throat insert intensely. It is difficult to ensure a stable aerodynamic shape even the fragmentation occurred if the throat insert has severe ablation, so it will has an bad effect on the thrust and efficiency of the engine.
With the application of throat insert becoming wider and wider, such as rocket boosters, long-range missiles, solid rocket engine and so on, and the more and more types of the metal additives, the materials properties for throat insert has been confronted with the higher requirements, which is also a key to the solid rocket technology developments. In general, throat insert materials used for solid rocket engine include refractory metal, graphite, carbon and carbon-based composite material, reinforced plastics and ceramic-based composite materials, etc. Among them, tungsten copper is one of the very suitable materials for throat insert fabricating. In addition, throat insert heat conduction can be divided into three steps: 1.Engine ignition instant impact heating; 2. Ablation when working steady; 3. The cooling process of flameout; wherein the thermal shock of throat insert often occurs at the moment of the ignition.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Thin Film Electrode Oxidation Glucose
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:59
Glucose exists in the nature by photosynthesis. Due to its abundant volume, low cost and reproducible, it is regarded as the main energy substrates to produce hydrogen. Glucose is the main waste of agriculture, food and paper-making industry, improper disposition will cause damage to environment. Recently many PEC systems produce hydrogen by glucose.
Tungsten oxide connect with electrocatalyst to produce hydrogen from glucose shows good photocatalytic activity, deposit electrocatalyst on the surface of photocatalyst can promote photocatalytic activity of semiconductor. Electrocatalyst deposited on the surface of semiconductor will form a layer of cover. By changing electron distribution in the system, the surface property of WO3 will be affected, so the photocatalytic activity is improved. Usually if Fermi level of WO3 is higher than the two combined material, electron will keep migrating from WO3 to depositing electrocatalyst. The shallow well potential Schottk energy barrier which can trap electron will form on the surface of metal and electrocatalyst. It provides effective trap potential for separating of photo electron and electron hole, it can resist the composite of photo electron and electron hole further, also the separating efficiency of charge carrier, thus to improve the quantum efficiency of photocatalyst.
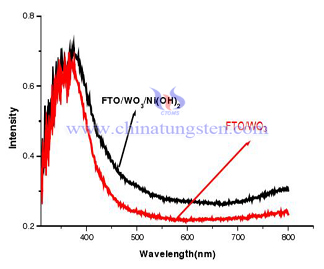
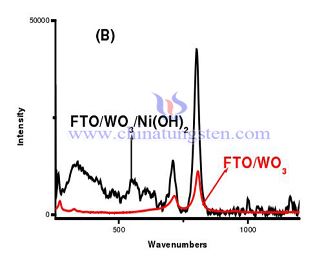
Use FTO/WO3/Ni(OH)2 thin film electrode in reduction of glucose experiment. Through this experiment, we can find that exposure of WO3 thin film electrode without Ni(OH)2 barely have photoelectrocatalytic glucose effect. Depositing Ni(OH)2 on the surface of tungsten oxide thin film can enhance photoelectric effect. Below is the raman spectrum and ultraviolet visible light absorption curve comparison of FTO/WO3 thin film electrode and FTO/WO3/Ni(OH)2.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Thin Film Electrode Optical Property
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:56
Below is the ultraviolet visible light absorption spectrum of WO3 thin film under different temperatures. PH value of precursory sol is 2.8, PEG content is 50%, heat treatment time is 3h. From the spectra, light absorption range of samples is almost the same which is below 4710nm. With the increase of heat treatment temperature, light absorption rage of thin film wave length ranges from 300~450nm increases. This is because the crystalline degree of sample increases with temperature which results in the improvement of light absorption efficiency.
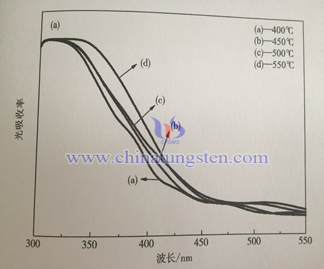
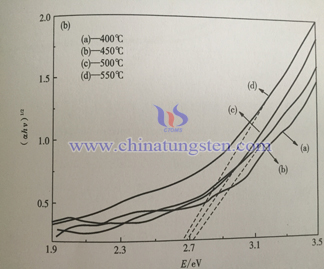
Below is the ultraviolet visible light absorption spectrum of sample with different adding amount of citric acid. Compared to the last graph, the absorption of all the samples is below 470nm, it is in accordance with WO3 theory band gap 2.7eV. With the increase of citric acid adding amount, light absorption of thin film increases within wave length 300~450nm, it is mainly because grain size and roughness increase on the surface of thin film. Nano crystalline scattering effect increases the spread distance of photon in film, it increases light absorption rate of thin film, it is benefit for light absorption efficiency.
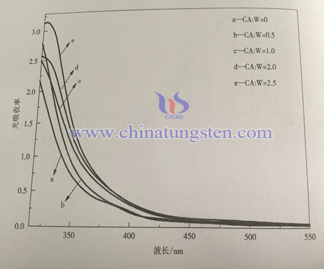
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Composite WO3/CdS/W Photocatalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:42

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
As-Reduced Ammonium Tungsten Bronze Nanoparticles Preparation
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:36


| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Roll Ring
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 16:57
Compared with the roll ring by other materials, tungsten carbide roll ring has many advantages, such as high hardness, high flexural or compressive strength, with low affinity of steel, low coefficient of expansion and excellent wear resistance and so on. What’s more, it can also effectively accelerate the rolling process, remarkably decrease stopping times and maintain high-speed rolling, which improves the overall efficiency; roll ring does not occur substantially scratches, it burns the melt and stick onto steel, and the shorter the time required for grinding; the rolled products have high dimensional accuracy, good surface quality, overall performance has improved significantly. According to differences of materials, tungsten carbide roll ring can be specifically divided into WC-Co based carbide, TiC based carbide and steel bonded carbide. In addition, in order to meet the special requirement of wear resistance and corrosion resistance, it can correspondly add some Ni, Cr elements. Generally, the content of WC of tungsten carbide is between 70%-97%, decrease the binder Co or the grain size of WC will all improve the hardness of roll ring.
The monolithic cemented carbide roll ring, for the user, a one-time investment costs are relatively high, which is also a barrier of development of carbide roll ring. So consider on the properties and the cost, researchers develop new tungsten carbide composite roll ring, which can greatly save the carbide consumption and decrease the production cost. It is composed of tungsten carbide outer ring and ductile iron inner ring. Tungsten carbide outer ring can ensure the roll ring has no deformation by high-speed friction under high temperature and high pressure, so the quality and dimensional accuracy of products will not be affected; ductile iron inner ring has high strength, good strength and ductility, which play an important role in rolling force delivering and can effectively reduce the failure rate of roll ring under impacting. Furthermore, it can also be processed by ductile iron and keyway with the keyway by a plurality of taper roller ring (up to 4) mounted on the roller body, compared to the overall carbide roller ring with the roll change a lot of convenience.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com