Composite WO3/CdS/W Photocatalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 14 June 2016 17:42
The meaning of compound semiconductor firstly lies in that the semiconductor particles with different energy band structure brings the possibility of narrow band-gap semiconductor sensitizing wide band-gap semiconductor nano-particles; secondly, in the quadratic element compound semiconductor, the energy difference between the two semiconductors making the photo-generated carriers injected into a energy level of the semiconductor particle from the another, thus to ensure the charge separation being effective and long-term; in addition, the excess charges which generated by coordination of different metal ions and different charge properties also help the increasing of the semiconductors capture proton or electronic, and thus to enhance the photocatalytic activity.
WO3/CdS/W composite photocatalyst is a complex phase of semiconductors, after the researchers continued to explore the photocatalytic reaction mechanism, and discussed the relationship between the composition of the composite catalyst, use level, pH of test solution, illumination time and the removal rates of COD, color. Experimental results have shown that, when in the conditions of the mass ratio of the WO3/CdS/W composite photocatalyst that m (WO3) / m (CdS) / m (W) equals to 60: 39: 1, the pH value of test solution is 6.5, the shinning time of 10h, the removal rate of COD, color of printing and dyeing wastewater reaches the highest point.

There are two forms of crystal of cadmium sulfide (CdS): α- formula presents in the form of lemon yellow powder; β- formula presents in the form of orange powder. High purity of CdS is a semiconductor with excellent property, which has a strong visible light photoelectric effect, and can be applied in the production like photoelectric cells, solar battery, light-sensitive resistors, photocatalyst ect.. Studies have shown that adding an appropriate amount of CdS into WO3 can improve the catalytic activity of the photocatalyst. That is because WO3 has the larger band gap of Eq = 2.8eV, and the CdS has the smaller band gap of is Eq = 2.12eV, the composite use of WO3 and CdS will substantially increasing the absorption rate of visible light.
At the same time, the use level of the reagent, pH value of test solution and illumination time also affect WO3/CdS/W the efficiency of photocatalyst:
1. With the increasing using level of reagent, the removing rate of COD, color increased; however, when it higher than a certain amount, the increase of the removal rate becomes very slow;
2. Under the premise of controlling the other variables being stable, the removal rate of COD, color increased with the increasing of pH value; however, when the pH value is higher than 6.5, the removal rate began to decline, and when the pH value equals to 6.5, its COD, color removal reaches the maximum, that we can see 6.5 is the optimal pH value;
3. Fix the amount of photocatalyst with the composition of m (WO3): m (CdS): m (W) equals to 60: 39: 1, the test solution pH value of 6.5, and just change the illumination time, then we can see that with the longer of the photocatalytic reaction time, the removal rate of COD, color increased gradually; but, when the reaction time is more than eight hours, the increasing rate of COD, color removal rate is becoming very slow; and when the reaction time reaches 10 hours, its COD, color removal rate reaches the highest value;
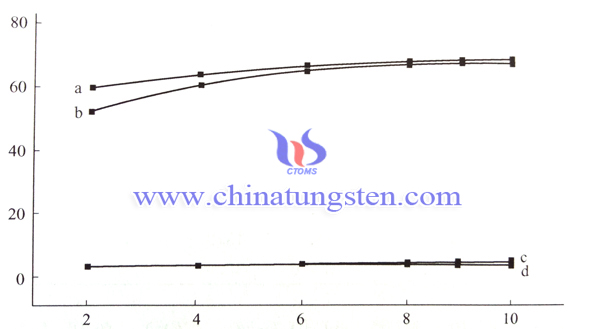
Wherein the abscissa represents the reaction time (h); the ordinate represents the removal rate (%); a, b, c and d are respectively on behalf of color removal efficiency of photocatalytic reaction, COD removal efficiency of photocatalytic reaction; COD removal efficiency of the dark reaction; COD removal efficiency of the blank experiment.
4. Furthermore, through the contrast of the dark reaction (the same amount of photocatalyst, but stirring reaction in the absence of light), the blank experiment (without catalyst, and stirring reaction under the light) and the photocatalytic reaction, we can draw that, photocatalytic oxidation reaction will not be occurred in the situations of simply adding the photocatalyst (only add photocatalyst, but the light is absence) and illumination condition (only light, but no photocatalyst), then it can be concluded that the photocatalytic reaction occurs only under condition of exist simultaneously of photocatalyst and light.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com