Rare Earth Doped Tungsten Electrode Introduction
- Details
- Category: Tungsten Information
- Published on Friday, 18 March 2016 12:00

Thoriated tungsten electrodes exists radioactive and long-term using will harm to humans and the environment, therefore developed rare earth doped tungsten electrodes having a similar performance with thorium tungsten electrodes use in welding field. Rare earth doped tungsten electrode having high melting point, low work function and good welding performance and gradually become the new favorite material in welding, cutting, thermal spraying and vacuum electric field.
Tungsten electrode doped with single rare earth oxides
Tungsten electrode doped with single rare earth oxides was first invented in the 1980s. It doped rare earth oxide La2O3, Y2O3, CeO3 into tungsten oxide after reduction, sintering and machining to produce a variety of tungsten electrodes. In practice, we found that this electrode have good arcing and welding property. Tungsten electrode doped with single rare earth oxide is suitable for small current welding. But in high current it service life is not long and the electron emission stability is poor.
Tungsten electrode doped with two rare earth oxides
Tungsten electrodes doped with two rare earth oxides were developed in the 1990s, primarily in order to improve the limitations of the single rare earth doped electrodes. Tungsten electrode doped with two rare earth oxides can carry large current and has large applications range. It has better overall performance than single rare earth doped tungsten electrodes. However, it has poor processing property.
Tungsten electrode doped with three rare earth oxides
Tungsten electrode doped with three rare earth oxides arcing performance, static characteristics and anti-burning property is superior to thoriated tungsten electrode. However, it has poor processing property.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Rare Earth Doped Tungsten Electrode
- Details
- Category: Tungsten Information
- Published on Friday, 18 March 2016 11:56
With the continuous development and progress of the welding industry, the reliability and stability of the electrode material is proposed more stringent requirements. Rare earth doped tungsten electrode with its high melting point, low electron work function, excellent thermal electron emission property, as the main material in place of thorium tungsten electrode. Besides, it successful replaces thoriated tungsten electrodes used in the small-scale welding. But in AC it has poor arcing property, short service life and other problems. In general, the arcing performance of the tungsten electrode doped with three rare earth oxides is superior to tungsten electrodes doped with single rare earth oxides. In rare earth doped tungsten electrode, ternary complex is better than binary complex.
In the late 1980s, a group of Japanese scholars have made some progress in the development of new tungsten electrode material, particularly in rare earth doped tungsten electrode material. They have operated a lot of work on electrode component design, performance comparison and mechanism study found tungsten electrode doped with two rare earth oxides can improve the performance of the tungsten electrode. For the binary rare earth tungsten electrodes, CeO2: Y2O3 = 1: 3 (CeO2 0.5%, Y2O3 1.5%), Ce2O3: La2O3 = 1: 1, La2O3: Y2O3 = 1: 3, has better performance and durability property. 1: 3 (La2O3 + Y2O3) tungsten electrode after 4h (180A) arcing still has good performance and arcing 10h durability remains better condition than single rare earth doped electrode.
For the ternary tungsten electrodes, the best rare earth oxide ratio is CeO2: La2O3: Y2O3 = 1: 1: 3. This electrode having good operating performance can sustain 10h (180A) operating. In rare earth doped tungsten electrodes, this electrode having low operating temperature, high electron emissivity, lowest electron work function and other properties, the overall performance is more prominent.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Cesium Tungsten Bronze Nanopowder in Transparent Insulation Coating
- Details
- Category: Tungsten Information
- Published on Thursday, 17 March 2016 17:29

| Tungsten Powder Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper PIM Degreasing Process (2/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 17 March 2016 17:04
Solvent degreasing refers to immersing billet heated in a solvent or solvent vapor, tungsten, copper blank soluble binder components by dissolution - diffusion dissolved in the appropriate solvent, so as to achieve the purpose of removing the binder. It can significantly reduce degreasing time and improve overall productivity; the amount of deformation is relatively small products, component distribution, which is an ideal degreasing powder injection molding method. However, solvent diffuses from outside to inside of tungsten copper blank, has less defects, but the solvent into the internal body, because it may cause excessive swelling of the sample deformation or cracking. After degreasing solvent, generally it needs to be dried to remove the body pores in the green solvent, wherein the remaining adhesive by thermal degreasing process. It also formed a two-step degreasing, namely solvent degreasing + thermal/heat degreasing.
For degreasing solvent control mechanisms can be divided into diffusion controlling, dissolution and diffusion controlling, dissolution controlling. Diffusion control is that when the binder is dissolved faster solvent with the binder dissolved long diffusion path, the corresponding diffusion is slow, which is in the diffusion phase, the V diffusion> V dissolved; dissolution and diffusion exist when the control is the dissolution rate and the rate of diffusion of the binder rather the case that V diffusion ≈ V dissolved; When the dissolution rate of the binder is less than the rate of diffusion of the solution, degreasing process is mainly controlled by dissolution, V diffusion <V dissolution at this time. Thereafter thermal degreasing, solvent degreasing process conducted among injection molded tungsten copper body has pulled out most of the wax, and paraffin prolapsed in internal body correspondingly left a void, which also heat degreasing rapid removal provided the conditions. Heat degreasing process is also can be divided into two steps, one is thermal decomposition process, a chemical reaction; another is the binder evaporating, a physical thermal mass transfer process.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper PIM Degreasing Process (1/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 17 March 2016 17:02
After material preparation and injection, the following steps are degreasing and sintering. Degreasing process is also known as forming agent or binder removal, which is the use of physical or chemical methods the added binder (such as alcohol, paraffin and the like) were removed. It needs the longest time of PIM and is the most difficult to control. Improper operation will cause the deformation or uneven component defects of tungsten copper blank, which will affect the final properties of tungsten copper products. The slow rate of degreasing and controlling difficulty has also hampered PIM development, so the related researchers attempt to develop new binder and new degreasing technology.
The common degreasing methods include heat degreasing, solvent degreasing, catalytic degreasing and siphon degreasing, and the siphon degreasing belongs to physical degreasing. As the name suggests is heated by thermal degreasing melting point difference, the binder evaporate escapes. It has broader applicability, cost is relatively low, the operation is simple and convenient, but a longer time is required for thermal degreasing, the production efficiency is low, and in the process is not easily controlled, prone to defects degreasing. Although siphon degreasing has high degreasing rate, the tightness between siphon material and tungsten copper blank can not be guaranteed and has higher cost so it is difficult to be popularized. Catalytic degreasing is a kind of chemical methods, its efficiency is relatively high, a small amount of deformation of the product obtained, but the acid-catalyzed vapor degreasing equipment used in corrosive and will pollute the environment.
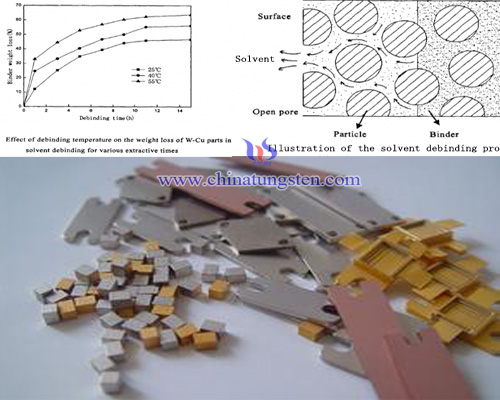
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
High Molybdenum Scheelite Concentrate Prepares Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Thursday, 17 March 2016 16:31
Over 70% ammonium paratungstate in China is produced by tungsten concentrate. Due to the consuming of tungsten concentrate over the past 50 years, tungsten resource structure has already changed:
1.Tungsten scheelite takes 73% of the existing tungsten resource.
2.Selective process is turning harder. For example, selecting of tungsten concentrate in Shizhuyuan which the WO3 grade is 45%, recovery rate reaches 80%. When WO3 grade is 65%, recovery rate is only 67%.
3.High impurity content. It is reported that tungsten mine reserve in Shizhuyuan reaches 25% in whole national industry reservation, the produced tungsten concentrate among which Mo/WO3>1.5%, more than 25 times of standard concentrate. Apart from this, impurities contents like As,P,Si,Sn are increasing.

Tungsten concentrate resource would run out in 10~15 years. In order to keep the sustainable development of tungsten industry, tungsten scheelite dealing method should be adopted gradually to replace tungsten concentrate. In the past, acid decomposition method is mainly used for dealing with tungsten scheelite, but it is insufficient in environment protection, product quality and recovery rate. Adopting NaOH decomposition method and selective precipitation method can leach WO3 from tungsten mine effectively. A large part of impurities would stay in the waste residue. Then combine traditional ion exchange method, removing impurities from tungstate which successfully solve the high molybdenum content, high impurities and calcium content in high molybdenum scheelite concentrate in the producing process.
The main steps: Firstly decompose tungsten mine material by NaOH, WO3 enters solution by means of Na2WO4. Impurities like P、Si、As are kept in the residue. These impurities are eliminated in Na2WO4 solution by ion exchange method. After it turns into (NH4)2WO4, precipitation agent M115 can be used to eliminate Mo and Sn. Then high purity APT can be obtained by evaporation crystallization. Molybdenum and precipitation agent can be recycled from molybdenum residue.
There is great economy and social efficiency by recycling molybdenum and precipitation agent from residue, especially when dealing with high molybdenum material. For instance, when dealing with tungsten scheelite in Shizhuyuan, 50~60tons of molybdenum can be recycled when producing 1,000tons of APT. The present recovery method is by leaching, let molybdenum into solution and keep precipitation in the residue which can keep the two things apart. Then molybdenum can be recycled from solution. Precipitation in residue can be sold as industrial raw material. Thus this method can lower the manufacturing cost, among which recycle and reuse of molybdenum is by no means a great fortune.
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
APT Prepares Negative Thermal Expansion Material-- Yttrium Tungstate Powder
- Details
- Category: Tungsten Information
- Published on Wednesday, 16 March 2016 18:07
Negative thermal expansion material means materials which are contrary to the common "thermal expansion" phenomenon, that is to say material shrinking when heated, while cooling down it expanding, which called "negative thermal expansion" effects (Negative Thermal Expansion, shorted for ΝΤΕ). ΝΤΕ material is widely used in precision controlling field, such as: in the aerospace high-precision assembly, all components are faced with extreme cold or hot environments, the use of ΝΤΕ material can eliminate the thermal expansion mismatch which is caused by temperature changes; telescope. Also the ΝΤΕ material is used in laser equipment, optical communication system, microelectronics ect.. Yttrium tungstate as the material with exceptionally NTE properties can be prepared from ammonium paratungstate (APT) and yttrium oxide.
Steps as bellows:
1. Generate tungsten trioxide powder from APT by conventional methods;
2. Uniformly mixing the yttrium oxide and tungsten trioxide powder by molar ratio of 1:3 by wet ball milling, and drying and grinding to obtain the raw material mixture;
3. The raw material mixture was placed in a muffle furnace for calcination treatment at 1100°C for 9 hours, then cooled down with the furnace, crushed and grinded to generate the calcined product;
4. Placing the calcined product in a muffle furnace under the same conditions for secondary firing (1100°C, 9 hours), and crushing, grinding after cooling down with the furnace, thus generate the secondary calcined product;
5. The secondary calcined product selected by a sieve with 300 meshes, then yttrium tungstate powder obtained.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Scraps Recycle APT--Acid Leaching Method
- Details
- Category: Tungsten Information
- Published on Wednesday, 16 March 2016 18:03
 Ammonium paratungstate (APT) being the important intermediate product of tungsten ore translating into tungsten products, its demand is growing in high-speed with the world economy development rapidly. Tungsten scraps are increasing at the same time. We present a method that directly translating tungsten oxide into ammonium tungstate by acid leaching method, and then carrying out crystallization to recycle APT. The steps are as follows:
Ammonium paratungstate (APT) being the important intermediate product of tungsten ore translating into tungsten products, its demand is growing in high-speed with the world economy development rapidly. Tungsten scraps are increasing at the same time. We present a method that directly translating tungsten oxide into ammonium tungstate by acid leaching method, and then carrying out crystallization to recycle APT. The steps are as follows:
1. Acid leaching and removing impurity: put the tungsten scraps into acid, reacting in the conditions that: acidity of 50-120g/mL, temperature of 60-100°C;
2. Drying and oxidizing: carrying out solid-liquid separation after the slurry acid leached and impurity removal, and then transferring into he drying furnace for drying after washing and purging, and transferring into the furnace for oxidation treatment at 600-1000°C after complete drying, now tungsten is oxidized into tungsten oxide;
3. Transformation: place tungsten oxide into ammonia solution, adding oxalic acid with the temperature heated and controlled at 80~150°C; set the transition pressure among 0.3~1.0MPa, keep stirring for 5-9 hours to give the crude acid ammonia solution, meanwhile trace the impurity elements Co, Ni, Fe, Ca ions into hydroxides and precipitation to separate;
4. Purification: determination the content of metal ion impurities Cu, Al, Mg and Ti, then adding ammonium sulfide, hydrogen peroxide for precipitation, separating to obtain pure ammonia tungstate solution;
5. Evaporation, crystallization and drying to obtain ammonium paratungstate products.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Gold-Plated Bracelet for Wedding Anniversary
- Details
- Category: Tungsten Information
- Published on Wednesday, 16 March 2016 17:58

| Tungsten Jewellery Supplier: Chinatungsten tungsten-jewellery.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
APT Prepares AMT- Thermal Degradation Method II
- Details
- Category: Tungsten Information
- Published on Wednesday, 16 March 2016 17:57

| AMT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com