Preparing Ammonium Paratungstate from Worn-Out Tungsten Oxide 1/2
- Details
- Category: Tungsten Information
- Published on Friday, 26 February 2016 16:36
Warn-out tungsten oxide including tungsten trioxide and blue tungsten oxide ect. which have been scrapped, and substandard thus must be reworked. This warn-out tungsten oxide which can not directly be used in tungsten smelting or other industries can be recycling. In this paper we will present a method for recovering ammonium paratungstate from warn-out tungsten oxide.

1. Handling the raw material
Screen the warn-out tungsten oxide by a 60~80 mesh sieve to remove caking tungsten oxide and mechanical inclusions; then the grinding caking tungsten oxide and also screen it;
2. Autoclaving with ammonia for preparing ammonium tungstate solution
1) Dilute the concentrated ammonia in deionized water or use water to absorb liquid ammonia, get ammonia with concentrate of 8~20%; and then formulating the slurry with stirring at the ratio that weight of tungsten oxide: volume of ammonia is 150~350g/L;
2) Add hydrogen peroxide when the material contains blue tungsten oxide, compress the kettle cover, heating to the pressure in the kettle is in the range of 4~l0kg/cm2 with stirring; the reaction time supposed to be 60~180 minutes;
3) Stop heating and cooling down to room temperature (20~40℃) after finishing the reaction; check if the color of solution is blue or not, if so, supplemented with hydrogen peroxide until it disappear; adjust the concentration of ammonia in ammonium tungstate solution among 3~5% with ammonia or water, and the concentration of WO3 is controlled among 120~350g/L;
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Studying Tungsten Trioxide Structural Phase Transition Law
- Details
- Category: Tungsten Information
- Published on Friday, 26 February 2016 16:32
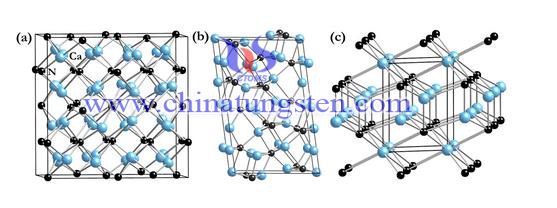 Structural phase transition region is divided into two basic types: remodeling and displacement type. Such classification is based on the grid by the formation of chemical bonds through periodic phase change. Strictly speaking, the non-reconstruction-type structural phase transitions are classified as displacement. Obviously, this is difficult to include atoms, molecules, cells and equivalent groups in a deep potential. For the reconstruction phase transition, we can include structural changes in carbon, graphite and diamond, which including the reconstruction phase transition and displacement phase transition, order - disorder phase transition, hybrid phase transition, and electron-phonon interaction associated phase transition. Of course, it does not include the superconducting phase transition, because its system structure.
Structural phase transition region is divided into two basic types: remodeling and displacement type. Such classification is based on the grid by the formation of chemical bonds through periodic phase change. Strictly speaking, the non-reconstruction-type structural phase transitions are classified as displacement. Obviously, this is difficult to include atoms, molecules, cells and equivalent groups in a deep potential. For the reconstruction phase transition, we can include structural changes in carbon, graphite and diamond, which including the reconstruction phase transition and displacement phase transition, order - disorder phase transition, hybrid phase transition, and electron-phonon interaction associated phase transition. Of course, it does not include the superconducting phase transition, because its system structure.
Structure of tungsten trioxide can be described as WO6 octahedron vertices that sharing three-dimensional network structure, however, WO3 symmetry is relatively low, because it is deformed over ReO3 structure. The lattice phonons and electronic structure of the combined effect of the changes is produced by several crystalline phases, these types of crystalline phases are the evolution from low to high symmetry with increasing temperature.
By studying the structure of the tungsten trioxide phase transformation, the phase change can be found in its crystalline order: monoclinic Pc (ε- WO3)→triclinic PT (ζ- WO3)→orthogonal Pbcn (β- WO3) →quartet P4 / ncc (α- WO3) → P4 / nmm, which presents different phase ramp in a different reaction temperature. In 2002, we found a new crystalline phase by continuous research. The high resolution neutron powder diffraction method confirms the existence of a new monoclinic phase at a temperature region of 720 ℃ to 790 ℃.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Versionhttp://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Inquiring Tungsten Trioxide Thin Film Translucent
- Details
- Category: Tungsten Information
- Published on Friday, 26 February 2016 16:21
 The proportion of the fabric sunlight penetrating, such as membrane light transmittance are 10-15%, which indicates that 10-15% of the sun can penetrate tensioned membrane, so that the building can save energy in the daytime without lighting. Reflection with the surface quality-related and coefficient scattering are the main factors. The density, grain boundaries and impurities are the main scattering. In the further non-ceramic cubic system, the presence of birefringence grain texture can be improved light transmittance.
The proportion of the fabric sunlight penetrating, such as membrane light transmittance are 10-15%, which indicates that 10-15% of the sun can penetrate tensioned membrane, so that the building can save energy in the daytime without lighting. Reflection with the surface quality-related and coefficient scattering are the main factors. The density, grain boundaries and impurities are the main scattering. In the further non-ceramic cubic system, the presence of birefringence grain texture can be improved light transmittance.
The tungsten trioxide thin film prepared by the sol - gel method , which shows its light transmittance is maximum and the corresponding wavelengths are 85.3%, 556nm; the peaks are and troughs are in the 350 ~ 600nm, the difference of the light transmittance at the peaks and valleys is 4.4%. WGZ-8 UV-V double beam of the incident light wavelength are 200 ~ 800nm, a light transmittance is 0% to 150% in spectrophotometer transmittance analysis.
And the use of magnetron sputtering method to prepare tungsten trioxide is different from the above, its maximum transmission rate and the corresponding wavelength ranges are: 90.1%, 572 ~ 582nm; peaks and troughs are 350 ~ 650nm, the difference between the peaks and troughs of the light transmittance is 21.2%. Comparison of the two lines shows that the difference between the two methods for preparing tungsten trioxide thin films is unlikely in the light transmittance. Thin films can be prepared by magnetron sputtering when the temperature at 450 ℃, the transmittance of the thin film is dropped by 8%. When the annealing temperature is 500 ℃, the transmittance of the thin film dropped by 8%, the peak position and peak shape are changed much.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Metatungstate Example in Oligomerization Process Producing Synthetic Lubricants
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 18:10

| AMT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Thermal Study on Electrospun Polyvinylpyrrolidone/Ammonium Metatungstate Nanofibers
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 17:56
When a too high heating rate and heating temperature (10 °C min−1, 600 °C) were used, the WO3 nanofibers completely disintegrated. At lower heating rate but too high temperature (1 °C min−1, 600 °C), the fibers broke into rods. If the heating rate was adequate, but the annealing temperature was too low (1 °C min−1, 500 °C), the nanofiber morphology was excellent, but the sample was less crystalline. When the optimal heating rate and temperature (1 °C min−1, 550 °C) were applied, WO3 nanofibers with excellent morphology (250 nm thick and tens of micrometer long nanofibers, which consisted of 20–80 nm particles) and crystallinity (monoclinic WO3) were obtained. The FTIR and Raman measurements confirmed that with these heating parameters the organic matter was effectively removed from the nanofibers and monoclinic WO3 was present in a highly crystalline and ordered form.
| AMT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide – Polycrystalline Diamond Buttons (2/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 17:40
Foreign scholars also try to use as cubic boron nitride carbide substrate and the buffer layer between the polycrystalline diamond layer having a volume ratio of 1 (CBN): 4 (polycrystalline diamond). The coefficient of thermal expansion of CBN at high temperatures between polycrystalline diamond and tungsten carbide, which effectively reduces the thermal stresses between them, and this excellent compatibility also reduces thermal stress micro-cracks caused by greatly enhancing the impact toughness of the composite buttons. Domestic research agencies by reducing the synthesis of polycrystalline diamond binder content, while increasing the pressure and increase the baking time between making polycrystalline diamond particles are bonded D - D chemical bond is increased, thereby increasing tungsten carbide - polycrystalline diamond compact buttons impact resistance.
D – D is a kind of chemical bond, which has higher binding strength than the physical strength of binder and the higher binding strength, the better impacting resistance. In addition, added polycrystalline diamond sintering process nickel-based or titanium-based additive, which has good wettability of polycrystalline diamond particles, can cover around the polycrystalline diamond particles in the sintering process, prompting more polycrystalline diamond melting the particles more conducive D-D bond combination.
American scholars designed a double polycrystalline diamond composite sheet, the outermost layer of polycrystalline diamond using coarse particles, with high impact resistance; and the inner layer using fine diamond particles, effectively improve the wear resistance. The inner layer of diamond, cobalt Co incorporated as a sintering agent, the outer layer of the coarse grain diamonds contain less or no cobalt; during sintering process, cobalt (Co) by the formula sweep extended through the inner layer to the outer layer of the fine particles of rough diamond grain diamond layer. Due to fine-grained diamond layer as the substrate, it has uniform distribution in the process of sintering and avoids undersintering and softening caused by Co distributed unevenly.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide – Polycrystalline Diamond Buttons (1/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 17:37
Tungsten carbide buttons have higher hardness and excellent impact ductility, which can be widely used in DTH drills for some rock drilling or impact rotary drilling operations. Based on the impact energy supplied by mud pumps, DTH drill uses buttons on the surface of the bit for rock drilling, which drilling depth up to 1000 meters or more, can be achieved in deep drilling and crushing complex formation, so it has strict mechanical properties to tungsten carbide buttons, especially impact resistance. But in some hard abrasive rock drilling, tungsten carbide buttons will be wore easily and leading to failure. Therefore, the process of tungsten carbide button with coatings has come into being.
Tungsten carbide – polycrystalline diamond buttons is a kind of new process, it uses tungsten carbide as substrate and coated with polycrystalline diamond. Tungsten carbide play a supportive role for the polycrystalline diamond, carbide and polycrystalline diamond has a higher hardness and wear resistance, but its impact resistance is lower, which has also become the focus of research related to researchers. In order to improve the impact toughness of tungsten carbide – polycrystalline diamond buttons, foreign researchers have proposed a functionally graded structure composite buttons, carbide diamond layer that is applied between the cemented carbide substrate and the polycrystalline diamond layer the buffer layer can effectively reduce internal stress between the polycrystalline diamond layer and the cemented carbide substrate; Or using polycrystalline silicon or silicon alloy diamond thermal expansion coefficient is closer to penetrate into the pores of pickled diamond, the experiments show that polycrystalline diamond toughness and fracture resistance capacity has been more significant improvement.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate Preparing Tungsten Rod 2/2
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 17:14
 Remarks:
Remarks:
1) The process conditions supposed to be: The temperature in the blue tungsten One-Reduction process may be: 530, 550, 580, 620, 650, 670℃, and the flow rate of hydrogen is 2.0~3.0m3/h, also the dew point is less than or equal to 60℃, boat loading amount is 300~350g, and the tungsten dioxide output will contain oxygen at rare of 12~14%;
2) The conditions of Secondary-Reduction are like bellows: temperature of 700, 750, 800, 830, 860, 860℃, the flow rate of hydrogen is 3.0~5.0 m3/h, the dew point is less than or equal to 60℃, boat loading amount is 280~320g.
5. Pickling
Washing the fine, medium and coarse tungsten powder respectively with the 5~6% concentration of hydrochloric acid and hydrofluoric acid to remove impurity;
6. Mix powders
Mix fine, medium and coarse tungsten carbide powder at the ratio of 20~25:50~60:20~25 in a mixer for 25 ~ 30min;
7. Forming at the condition of cold isostatic pressing
8. Sintering at high temperature
Sintering the tungsten billets which is pre-sintered in a vertical melting machine under the protection of hydrogen, set a certain conditions, then we get the tungsten which is needed.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate Preparing Tungsten Rod 1/2
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 17:09
Tungsten Halogen Lamps is mainly applied in the lighting system of automotive, electronic and aerospace industry. Due to the special using environment in which the shock and vibration presence, thus the core materials-tungsten filaments is required to be with good recrystallized of dovetail joint structure, also the ratio of length and width is supposed to larger than 12, which means it has the good properties of sagging resistance at high temperature and deformation resistance. Therefore, improving the properties of tungsten bars which can improve the quality of Tungsten Halogen Lamps has become extremely urgent. A literature has proposed a method for preparing tungsten rod with good properties of sagging resistance at high temperature and deformation resistance which can applied in the production of Tungsten Halogen Lamps.
The specific steps are as follows:
1. Mix monoclinic and spherical ammonium paratungstate by the ratio of 3:1~1.5, as the raw materials;
2. Pre-reduction
Make the raw material going through a reducing furnace with four temperature zone, reduction to generate blue tungsten oxide at the hydrogen atmosphere;
3. High Potassium doping
Mix the blue tungsten oxide into a solution containing with potassium silicate and aluminum nitrate;
4. Reduction
1) Using Secondary-Reduction to generate fine grain tungsten powder with FSSS 2.0~2.6um from doped blue tungsten;
2) Generate coarse tungsten powder with FSSS 3.4~4.0um by One-Reduction method;
3) Generate medium grain tungsten powder with FSSS 2.7~3.3 um in a six temperature zone furnace by Secondary-Reduction method;
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
SEM Technique Analyzing Tungsten Trioxide Nanowire
- Details
- Category: Tungsten Information
- Published on Thursday, 25 February 2016 16:02
 Structural Equation Modeling is a blend of multivariate statistical techniques of factor analysis and path analysis. Its strength lies in the quantitative study of the interactive relations among multiple variables. In nearly three decades, SEM is used in a large number of social and behavioral sciences, in the recent years; it has been increasingly used in market research. By SEM technique analyze of tungsten trioxide nanowire, we have a better understand that the impact of changes in external factors caused by the topography. It can be analyzed by the precursor and the reaction temperature.
Structural Equation Modeling is a blend of multivariate statistical techniques of factor analysis and path analysis. Its strength lies in the quantitative study of the interactive relations among multiple variables. In nearly three decades, SEM is used in a large number of social and behavioral sciences, in the recent years; it has been increasingly used in market research. By SEM technique analyze of tungsten trioxide nanowire, we have a better understand that the impact of changes in external factors caused by the topography. It can be analyzed by the precursor and the reaction temperature.
Nanowires may be defined as having in the transverse direction that is limited to one-dimensional structure at 100 nm (without longitudinal limit). Nanowire suspension means the end of the nanowire under vacuum. Depending on the composition of the material, the nanowire can be divided into different types, including metallic nanowires, nanowires and the insulator semiconductor nanowires.
The preparation of tungsten trioxide nanowire can be divided in three steps. Firstly, obtaining the diameter about 200-500nm tungsten trioxide nanowire, the length about 5-10μu with 1.3g of sodium tungstate reaction in the same conditions. Secondly, we can also study its effect on the appearance of the tungsten trioxide nanowires synthesized by controlling the reaction temperature. Finally, we obtain tungsten trioxide nanowires in the uniform conditions that the reaction time is 32h at 240 ℃, and its diameter is 200-500nm, its length is about 5-10μm.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com