Energy Storage Type Tungsten Trioxide Photocatalyst
- Details
- Category: Tungsten Information
- Published on Friday, 27 May 2016 17:33
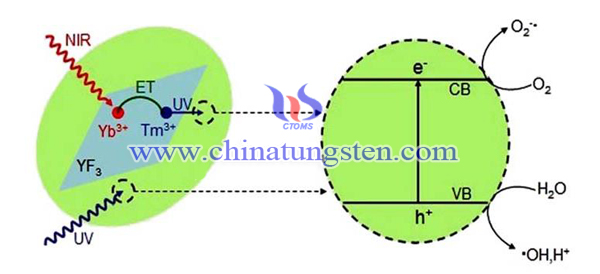
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nano Pt Supported Tungsten Trioxide Photocatalyst
- Details
- Category: Tungsten Information
- Published on Friday, 27 May 2016 17:31
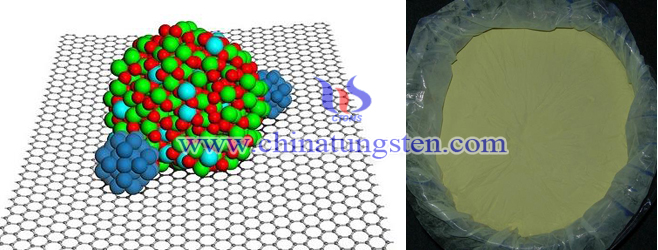
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Bar and Tungsten Wire
- Details
- Category: Tungsten Information
- Published on Friday, 27 May 2016 10:06
At present, tungsten filament is mainly used as electric light source of electric incandescent lamp, halogen tungsten lamps and others. When using as a luminous body of the filament lamp, a small amount of potassium, silicon and aluminum oxides should be added during the smelting process. This kind of wolfram filament is also known as doped tungsten wire and 218 wolfram wire or anti-drooped tungsten filament. Uniformity condition of density of tungsten bar plays an important role in the quality of tungsten product. The number, size and distributing condition of holes of wolfram bar not only have a greater impact on its high-temperature performance, but also affect the mechanical properties.
Processing tungsten round bars and square bars made from the same batch of tungsten powder raw material. After stretching them to 500W finished wire, using Japan or West Germany crack flaw detectors to detect 50000m wolfram filament respectively. By the force of contrast, it can be found that, the total length of the crack of tungsten wire prepared by round tungsten bar is about 0.98% to 1.20% of. On the other hand, for the same specification of tungsten square bar, crack accounts for 4.21% ~ 58.82% of the entire length of wolfram filament. Obviously, the crack of tungsten filament made by tungsten round bar is much smaller than square bar. Tungsten is widely used as refractory metals. In wire form, wolfram wire is essential for the production of lighting products and other goods where its high temperature properties are of use. Its properties are a melting point of 3410° C, a low coefficient of thermal expansion and low vapor pressure at elevated temperatures along with good electrical and thermal conductivity.
From the perspective of wire-wound performance, under the same conditions, the wire-wound performance of wolfram filament prepared by round bar is better than the square one. According to the spiral resilience, we can see that the helical filament of the tungsten round wire would not crack in the stretching process, and the distance of the pitch is relatively equal. Under the same situation, square spiral filaments have the flerry phenomenon.
All in all, any non-uniformity processing of wolfram bar will cause the deformation of tungsten material, leading to its internal organization become non-uniformity, causing breakage of the inner wall of wire, especially at the junction of uneven tissue, which is more easily to have a crack.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Waste Tungsten Bar and Tungsten Powder
- Details
- Category: Tungsten Information
- Published on Friday, 27 May 2016 10:04
Tungsten powder is in the form of powder of tungsten metal, which is the raw material of manufacturing process of wolfram, tungsten alloy and tungsten products. Pure tungsten powder can be processed into wire, rod, tube, plate and any other shapes. If wolfram powder mixes with other metal powders, it can be made into a variety of tungsten alloys, such as tungsten-molybdenum alloy, tungsten-rhenium alloy, tungsten-copper alloy and high density tungsten alloy. Another important application of tungsten powder is to make tungsten carbide powder, and then the produce cemented carbide tools, such as lathe tool, hobbing, drilling bit and die. Pure tungsten bar includes tungsten rod, tungsten steel bar, tungsten rod, tungsten sintered bars and more, which is mainly used to found ingredient of material, cutter and heads, tungsten wire for lights instruments, electric contact points and conductor of heat, crankshaft and cylinder barrel of advanced automobile, ingredient of kinds of heat-resistant steel.
People are used to abstract the metal from the ore, however, recycling and reproduce discarded metal can not only save energy, reduce costs, but also help to improve the ecological environment. Therefore, this method has attracted serious concern in the world. Especially in Japan, the country takes the project as the development of "second mining", which contains tungsten steel scrap, tungsten metal and its alloy scrap, cemented carbide scrap, grinding debris, dust and other secondary raw materials. Many countries have begun to set up a special department in charge of recycling the waste tungsten. From example, the Soviet Union has established a research institute in charge of the recovery of non-ferrous metal scrap. Through a series of industrial tests, a tungsten-containing waste treatment process developed by the institute is considered as the most promising step. In recent years, many countries are researching and developing how to deal with the waste tungsten bar. The ways to handle waste wolfram bar and reproduce wolfram include electrolytic process, zinc process, high temperature oxidation, chemical dissolution and chlorination pulping process.
It is proved that waste end block of wolfram powder can be used as a raw material instead of industrial wolfram powder, and it can produce the same quality of ultrafine particles. This can greatly reduce the industry costs. What’s more, the most important is the reasonable reuse of resources that can improve evils caused by the rapid development of industry.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Rare Earth Affected Tungsten Electrode Sintering Process
- Details
- Category: Tungsten Information
- Published on Thursday, 26 May 2016 19:51

The content of rare earth in tungsten powder at sintering process will affect tungsten electrode sintering temperature and density. And the main reason is due to the dispersal of rare earth in sintering process. As we know, with increasing of rare earth content, the influence of rare earth on tungsten grain is increase. On the one hand, rare earths under heat effect will have diffusion migration occur, and resulting in the distribution of rare earth on tungsten powder is uneven. On the other hand, there is hindered effect of rare earth on tungsten grains growing. So containing large rare earth area sintering is more inadequate. On the contrary, the area where has small rare earth content can completely be sintered. And this is the reason that tungsten grain size has large difference. And part of pores is blocked, resulting in density to reduce. Causing by heat effect the rare earth unevenness will be more severe, and the sensitivity of the process is also more obvious.
One of performance of process sensitivity is forming clear stratification. The core grain of tungsten blank bar has grown significantly, while the blank edges structure is finer. Observing by SEM image, you can see the internal organization is thick and has high degree crystallinity. But the edge organizations of tungsten blank are showing particle characteristics and organization is small. What’s more the crystallinity has low degree.
To employ electron probe microanalysis on the fracture surface of tungsten blank bar which is after ground and polished and t has obvious delamination phenomenon. Form the edge to the line of the core of tungsten blank bar, we can see, there are the tungsten blank which has delamination phenomenon the ingredients may also different. At the junction of the two layers, the higher rare earth content, the structure is coarser. On the other hand, fine structure has low rare earth content. And the reason may be because sintering process operates at flowing hydrogen atmosphere. The hydrogen will take the heat for the surface of tungsten blank bar away, so that the surface temperature of the blank is lower, and the center temperature is higher, so there is a temperature gradient in a radial direction of tungsten blanks. Rare earth at this temperature gradient effect diffusion and migration is faster, especially when high temperatures are very high, and migration of rare earth is quickly. While at higher temperatures, the sintered degree of tungsten electrode also increased. The area has large rare earth content will hinder the tungsten grain growth and the effect is large. Low rare earth content area the effect is smaller. For the central area, the lower rare earth content, but has high temperatures, then in this region tungsten powder will preferentially to form coarse crystal grains. For the edge region, there has lower rare earth content, and the temperature is low, so only sintered to a higher temperature this part can be fully sintered. The junction of these two regions has high rare earth content, so there are large hinder effect on grain growth, and the temperature range is between these two area, so that the combined effect making the grains in this area is relatively small, which is similar to edge are. In view of this conclusion is the premise of tungsten is good conductor of heat and has fast heat conduction.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Rare Earth Affected Tungsten Electrode Sintering Process - Temperature and Density
- Details
- Category: Tungsten Information
- Published on Thursday, 26 May 2016 19:44
For powder sintering, the process is generally divided into three phases. The first phase is the initial sintering, called bonding stage. In this phase due to the temperature rise, the atomic amplitude of contact point between the particles is increase and there will have diffusion occurs, making particle contact change from point to the surface. And inner grains of particle are not change. Besides, the particle shape has little change and sintered blank is no shrinkage. What’s more sintered blank density changed little, but the strength and conductivity of it has increased significantly.
The second phase is sintering neck growth and produce sticky plastic flow stage. In this stage, the temperature is higher, so the migration rate of atoms phase junction surface is increase. And migration rate increase makes sintering neck to grow up and particle distance to reduce, forming the associated pore network. Besides, the grains in the particles grow up and grain boundaries moves over the pores. What’s more, the placed where grain boundary swept the pores disappear, so in this phase sintering blank has obvious shrinkage phenomenon and density and strength has significantly enhanced.
The third stage is closed pores spheroidization and shrinking stage. At this stage, most of the pores are completely divided, so closed-pore number has increased considerably and the shapes of pores tend to spherical and shrinking. Besides, at this stage, the entire sintered body continues to shrink. Closed-pore number has increased and shape tends to spherical achieved by pores disappearing and pores quantity reducing. What’s more, this stage can last for a long time.
The core process of sintering is the material migration. According to the migration patterns the powder sintering mechanism can be divide into there types which is diffusion mechanism, evaporation mechanism and condensation flow mechanism.
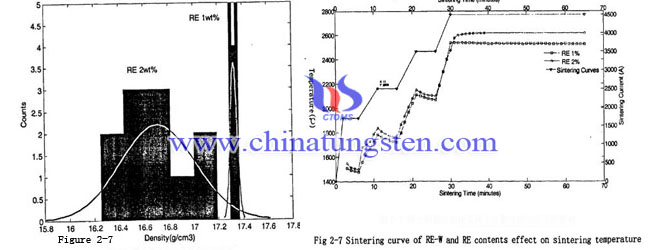
Analysis at the same sintering process, different rare earth content tungsten powder sintered, to understand the role of rare earth in tungsten powder sintering process and its influence on the tungsten electrode. The sintering curves and temperature change of different content rare earth tungsten billet shown in Figure 2-7. From the Figure 2-7 you can see at the same sintering current, rare earth content increase will lead to sintering temperature rises. And the reason is due to the rare earth and other impurities content increased, the electron scattering effect increased as well, causing the resistivity of the sintered tungsten bar increased. For constant current fusion sintering verification, the performance is the temperature also rises.
To test density of many tested sintered blank found the density is normal distribution, showing in Figure 2-8. From Figure 2-8 we can see two type tungsten powders which have different rare earth content in the same sintering process, the density has significantly different. Rare earth content with 1%, the tungsten powder after sintered has large density and has narrow distribution. The standard deviation is about 0.028. Tungsten powder with 2% rare earth content in the sintering process has lower density and has wide distribution. The standard deviation is about 0.303. Description in this process, tungsten electrode blank has strong sensitivity and production stability is difficult to control.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Photocatalyst - Air Purification Master
- Details
- Category: Tungsten Information
- Published on Thursday, 26 May 2016 16:17
 Photocatalyst are materials that induce photocatalytic reaction under photoirradiation. A general definition of photocatalysis, a conceptual name of photocatalytic reactions, is a chemical reaction induced by photoabsorption of a solid material, or “photocatalyst,” which remains unchanged during the reaction. With the promotion of Industrialization process progresses continue, more and more urban residents living in an environment with the extremely high of air pollution index. In recent years, since the striking of haze, the indoor air purification has caused more people's attention. Because of the explosive growth of air purification demanding, photocatalytic product in the market emerge in endlessly. Wherein, the most representative product is photocatalyst which leading by titanium dioxide, that is the champion with its superior performance.
Photocatalyst are materials that induce photocatalytic reaction under photoirradiation. A general definition of photocatalysis, a conceptual name of photocatalytic reactions, is a chemical reaction induced by photoabsorption of a solid material, or “photocatalyst,” which remains unchanged during the reaction. With the promotion of Industrialization process progresses continue, more and more urban residents living in an environment with the extremely high of air pollution index. In recent years, since the striking of haze, the indoor air purification has caused more people's attention. Because of the explosive growth of air purification demanding, photocatalytic product in the market emerge in endlessly. Wherein, the most representative product is photocatalyst which leading by titanium dioxide, that is the champion with its superior performance.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Titanium Dioxide-Tungsten Trioxide Photocatalyst Composite Film
- Details
- Category: Tungsten Information
- Published on Thursday, 26 May 2016 16:15
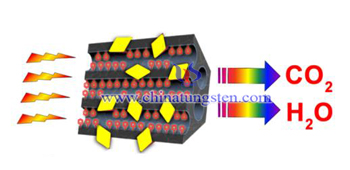 Photocatalyst material has been serious attention in recent years, due to it has oxidative decomposition reaction and the hydrophilic phenomenon. If the material is coated on the window glass in a large area, it is possible to increase the reaction area, and thus will bring more effective decomposition of pollutants or harmful gases; in addition, its super-hydrophilic phenomenon can make the glass always remain clean. Photocatalyst as a nano-scale transition metal oxide, the outdoor illumination of UV (ultraviolet) and indoor lighting which below 400nm band can stimulate it to produce catalytic reactions in general, without the need for additional waste of energy.
Photocatalyst material has been serious attention in recent years, due to it has oxidative decomposition reaction and the hydrophilic phenomenon. If the material is coated on the window glass in a large area, it is possible to increase the reaction area, and thus will bring more effective decomposition of pollutants or harmful gases; in addition, its super-hydrophilic phenomenon can make the glass always remain clean. Photocatalyst as a nano-scale transition metal oxide, the outdoor illumination of UV (ultraviolet) and indoor lighting which below 400nm band can stimulate it to produce catalytic reactions in general, without the need for additional waste of energy.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Bronze Application in Ceramic Capacitors
- Details
- Category: Tungsten Information
- Published on Wednesday, 25 May 2016 18:21

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nano Precious Metal Modified Tungsten Trioxide Photocatalyst Coating
- Details
- Category: Tungsten Information
- Published on Wednesday, 25 May 2016 18:06
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com