Tungsten Alloy Radiation Shielding For X-ray Machine
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 17:58
 An X-ray machine is a device used to generate X-rays. It is made up of a control console which enables the x-ray technician to choose a variety of x-ray techniques suitable for that specific exam, an X-ray generator that creates and produces the desired KV, MA and X-ray tube. The X-ray tube, like any vacuum tube, contains a cathode, which directs a flow of electrons into a vacuum, and an anode, which collects the electrons and is made of tungsten to disperse the heat generated by the collision. When the electrons collide with the target, about 1% of the resulting energy is emitted as X-rays, with the remaining 99% released as heat. Due to the high energy of the electrons that reach relativistic speeds the target is usually made of tungsten even though other materials can be used particularly in XRF applications. The types of X-ray machine include the portable medical X-ray system, the industrial X-ray detection machine and the belt detection X-ray machine. X-ray machines are widely used in hospitals to help doctors diagnose the disease, in industry for non-destructive testing and in railway stations and airports to carry out security checks.
An X-ray machine is a device used to generate X-rays. It is made up of a control console which enables the x-ray technician to choose a variety of x-ray techniques suitable for that specific exam, an X-ray generator that creates and produces the desired KV, MA and X-ray tube. The X-ray tube, like any vacuum tube, contains a cathode, which directs a flow of electrons into a vacuum, and an anode, which collects the electrons and is made of tungsten to disperse the heat generated by the collision. When the electrons collide with the target, about 1% of the resulting energy is emitted as X-rays, with the remaining 99% released as heat. Due to the high energy of the electrons that reach relativistic speeds the target is usually made of tungsten even though other materials can be used particularly in XRF applications. The types of X-ray machine include the portable medical X-ray system, the industrial X-ray detection machine and the belt detection X-ray machine. X-ray machines are widely used in hospitals to help doctors diagnose the disease, in industry for non-destructive testing and in railway stations and airports to carry out security checks.
X-ray is a form of electromagnetic radiation. Most X-rays have a wavelength ranging from 0.01 to 10 nanometers, in line with frequencies in the range of 30 petahertz to 30 exahertz and energies in the range of 100 eV to 100 keV. The wavelength of the X-ray is shorter than that of UV ray and typically longer than that of gamma ray. In many languages, X-radiation is referred to with terms meaning Röntgen radiation, who is usually credited as its discoverer, and who had named it X-radiation to signify an unknown type of radiation. X-ray has penetrating effect, ionizing effect and thermal effect. In medicine it is often used for fluoroscopy, in industry for industrial flaw detection. Since the X-ray has a strong penetrating effect, when a living organism is irradiated with X-ray, its living cells can be inhibited, destroyed and even killed, resulting in varying degrees of changes in physiology, pathology and biochemical aspects. In the use of X-ray technology, it also found that X-ray can lead to the patient hair loss, skin burns and can cause vision disorder, leukemia and other ray injury problems on the related personnel.
Tungsten alloy radiation shielding can be used to shield X-ray generated by the X-ray machine. Compared with conventional shielding materials (such as lead), tungsten alloy shielding reflects good value. Since the tungsten alloy has a high density, under the condition of the same thickness, the radiation shielding ability of tungsten alloy is twice than that of lead shielding, and the weight of tungsten alloy shielding is 25 to 50% less than lead. In addition, lead is toxic and tungsten alloy shielding is non-toxic so that it will not harm the human body or pollute the environment.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Bronze Structure Materials Application in TBC
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 17:50
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Wire Process
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 16:48
The basic process of tungsten copper wires is : Mixing → molding or isostatic pressing → infiltration, sintering tungsten copper rod or tungsten copper block → machining. Except conventional infiltration process, there are some popular processes used more, such as high-temperature liquid phase sintering and activated liquid-phase sintering. The principle of infiltration is that porous tungsten skeleton wetted by copper liquid, under the capillary force the copper liquid flows along the W particle gap, filling the pores of porous tungsten skeleton. So tungsten copper materials have higher density, excellent sintering properties and electrical conductivity; High temperature liquid phase sintering since the sintering temperature is high, a long time, will make a lot of volatile copper phase, so that the sintered tungsten copper material density decrease, the performance will be subject to different degrees of impact, it is difficult to obtain a high density, high conductivity tungsten copper alloy material; activated liquid phase sintering improves the relative density, the hardness and tensile strength of tungsten copper by adding small amounts of trace elements (Ni, Fe, Pd, etc.). But on the contrary, the activated elements will dramatically decrease the electrical and thermal conductivity, which is not suitable for the occasions with high requirements of the conductivity.
The technologies to machine tungsten copper rod or block in wires include drawing processing, rolling processing, roller die drawing techniques and rotary forging technology. Drawing processing take advantage of metal plasticity, when working in the external force so that it is forced through the die, the metal cross-sectional area is compressed to obtain the desired cross-sectional shape and size of the processing method. Preparation of tungsten and copper wire by repeatedly hammering and drawing forming, drawing conducted at room temperature, metal products will produce obvious hardening.
But for tungsten copper with lower Cu content, it has poor plasticity, the drawing rate received extremely limited, which is difficult to draw molding; rolling process is to rely on the friction two rotating rollers and the rolling member rolling pulled into the roll gap, it is compressed to produce plastic deformation process. In addition, the rolling process can also refine the grain, improve the organization, can significantly improve the mechanical properties of the metal alloy, suitable for mass production; Roller die drawing process the billet is drawn in a non-grooved drive, freely rotating rollers consisting of, the sliding friction with the material of the die orifice was changed to rolling friction bearing, the stretching process is more effort not only suitable for drawing round wire, shaped wire may be stretched, but not suitable for low plastic material.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Wire Description
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 16:46
Tungsten copper alloy is a kind of psudoalloy, which consists of the hard phase W and the binder phase Cu two immiscible metals, so it only can be fabricated by PM (Powder Metallurgy). Tungsten copper material has both advantages of tungsten and copper, the high density, high melting point, excellent wear resistance and corrosion resistance of W; the excellent electrical and thermal conductivity, good plasticity of Cu; and at a temperature above the melting point of copper alloy of copper liquefied evaporated to absorb a lot of heat, reducing the surface temperature. So tungsten copper material is also known as heat sink materials.
According to the shapes and applications, tungsten copper products can be specifically divided into tungsten copper rod, tungsten copper block, tungsten copper plate, tungsten copper contact, tungsten copper tube, tungsten copper electrode, tungsten copper heat sink, tungsten copper wire and so on. In the early 1960s, tungsten copper wire and tungsten copper electrode has been used in EDM (Electrical Discharge Machining) and resistance welding because of its high density, high strength, excellent electrical and thermal conductivity and arc ablation resistance. So far, with the developments of relevant technologies and further study of researchers, tungsten copper begin to use in plasma electrode material processing, precision machining, spraying, LED and other electrode fields. But since the special PM structure of tungsten copper, it has been greatly limited used in wire. In order to improve the uniformity, mechanical properties and production cost, it needs to looking for new technologies and processes.
Used in EDM electrodes of tungsten copper wires are supposed to meet the following requirements:
1. On the basis of excellent electrical and thermal conductivity, it also has good resistance to spark erosion;
2. Good structure uniformity and density, in order to ensure the stability of the electrical processing and improve the utilization of electrode material;
3. It has lower machining consumption and dose not affects the quality and the overall efficiency;
4. Easy for molding, machining molds and products according to design requirements to provide a correspondingly shaped profile tungsten copper electrode bars or complex shapes.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Contained Vehicle Denitration Catalyst without Vanadium
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 16:09

However, when SCR catalyst containing vanadium used for cleaning motor vehicle exhaust, it may lead to toxic vanadium compound going into the air because of the volatility of vanadium under high temperature, thus to cause environmental pollution and personal injury. So, the listed capacity of the car catalyst containing vanadium is low. Therefore, for a long time, researchers and vendors are ongoing committed to provide no vanadium SCR denitration catalyst.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Titanium Based Tungsten Trioxide Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 16:07
 Titanium is a silvery-white transition metal, which is considered as a rare metal, with the properties of light-weight, high-strength, metallic luster, moisture chlorine corrosion; its most common compound is titanium dioxide, which is often used as the carrier of denitration catalyst. The typical plate denitration catalyst is based on stainless steel and coated with a catalyst paste (ingredients: titanium dioxide, vanadium pentoxide, tungsten trioxide and molybdenum trioxide, etc.).
Titanium is a silvery-white transition metal, which is considered as a rare metal, with the properties of light-weight, high-strength, metallic luster, moisture chlorine corrosion; its most common compound is titanium dioxide, which is often used as the carrier of denitration catalyst. The typical plate denitration catalyst is based on stainless steel and coated with a catalyst paste (ingredients: titanium dioxide, vanadium pentoxide, tungsten trioxide and molybdenum trioxide, etc.).| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Testing Standards of Carbon Sulfur Analyzer and Tungsten Granule——Test Conditions and Test Methods
- Details
- Category: Tungsten Information
- Published on Thursday, 19 May 2016 10:47
Except the technical requirements, testing standards of carbon sulfur analyzer and tungsten granule also include - test conditions, test methods, management of the results of treatment, re-testing period. The relative standard deviation of carbon is lower than 1.0%, sulfur should be lower than 4.0%, the analysis time should less than one minute, the weighing stability of less than 0.002 grams. Environment conditions for testing should meet the standards. The ambient temperature should be controlled between 15 ℃ to 30 ℃, relative humidity must be less than 80%. If it is measured against the amount of 0.0010% to 0.0100% of the carbon and sulfur, the relative humidity should be less than 60%. Power supply is (220 ± 4.4) V, (50 ± 1) Hz, and it should be ensured that the detection environment has no strong vibration, no strong electric, no active gases and without any impede of magnetic fields.
The main test equipments and materials for detection should be in accordance with national standards. Using the approved the steel components by the state metrological administrative department to analyze national primary, secondary reference material. Uncertainty of the determination of carbon and sulfur less than one-third of error of indication, weighing with 1g standard weights. According to visual indication and touch to detect the appearance of instrument. The instrument is required to be preheated on the basis of instructions before detecting. Select carbon content within the range from 0.100% 1.000%, and choose sulfur content within the range from the 0.010% to 0.100%, weighing 0.5 g of sample every time, repeated detecting for 7 times, calculating the standard deviation and relative standard deviation in according with the standard formula calculation method . Weighing with 1 g standard weight, continuously weighing for six times, the difference between the maximum and the minimum is called weighing stability. After the test is completed, certificate of qualification can be sent, if unqualified, pointing out that the failed project. Test cycle usually two years. While moving, repairing or doubting on the measurement results, users can test the instrument.
If the carbon sulfur analyzer used by the company are not included in the mandatory verification of measuring instruments catalog, on the other hand, these instrument are not applied in trade settlement, safety, health, environmental monitoring, there is no need to test it compulsively.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
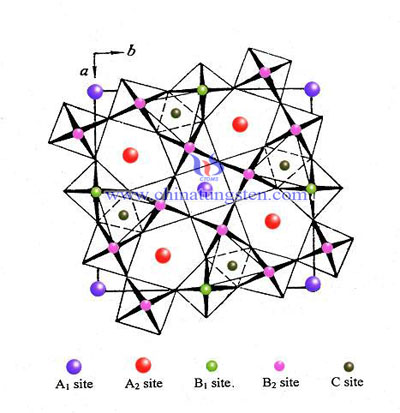
Cubic Hydrogen Tungsten Bronze Crystal Structure
- Details
- Category: Tungsten Information
- Published on Wednesday, 18 May 2016 18:17
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Glass Furnace Flue Gas SCR Denitrification Catalyst Uses Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 18 May 2016 16:56
 There are many sources of nitrogen oxides, in which glass furnace flue gas is the main one. The mass of nitrogen oxides in the glass furnace flue gas emissions is up to 14 million tons per year. Furthermore, with the advance of modernization, the consumption of housing and car growing; plus the new growth point of solar glass industry, all these resulting in increasing demand for glass, glass industry continues to develop, thus nitrogen oxide emissions rise.
There are many sources of nitrogen oxides, in which glass furnace flue gas is the main one. The mass of nitrogen oxides in the glass furnace flue gas emissions is up to 14 million tons per year. Furthermore, with the advance of modernization, the consumption of housing and car growing; plus the new growth point of solar glass industry, all these resulting in increasing demand for glass, glass industry continues to develop, thus nitrogen oxide emissions rise.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Waste Plate Denitration Catalyst Recycles Metal Oxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 18 May 2016 16:53
 The main treatment methods of waste denitration catalyst currently are landfill, burning and recycling, the first two methods have huge potential dangers on the environment. In addition, due to full implementation of emission reduction, the waste catalyst generated increasingly; the treatment ways of landfill and incineration will not only occupy a lot of land, but also bring a secondary pollution to the environment. The way of recycling includes used as boiler slag recycling vulcanizing agent and raw materials recovery, thus to apply for the production of new catalyst, the raw materials of steel plants; wherein, heavy metal with toxic and its relatively components must be separated. The way of basic recycling titanium, vanadium and tungsten from tungsten trioxide waste plate denitration catalyst is as follows:
The main treatment methods of waste denitration catalyst currently are landfill, burning and recycling, the first two methods have huge potential dangers on the environment. In addition, due to full implementation of emission reduction, the waste catalyst generated increasingly; the treatment ways of landfill and incineration will not only occupy a lot of land, but also bring a secondary pollution to the environment. The way of recycling includes used as boiler slag recycling vulcanizing agent and raw materials recovery, thus to apply for the production of new catalyst, the raw materials of steel plants; wherein, heavy metal with toxic and its relatively components must be separated. The way of basic recycling titanium, vanadium and tungsten from tungsten trioxide waste plate denitration catalyst is as follows:| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com