Doping Tungsten Trioxide Gas Sensing Properties Study
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 18:07
The gas sensor is a sensitive device that can be capable of sensing the concentration of the gas in environment. It can convert into electrical signal according to the intensity of these electrical signals from information about the type of gas and its concentration. Stable gas sensors have the ability to distinguish between gas, strong gas sensitivity, fast response signal detection, long life and other characteristics. Tungsten trioxide material has been widely studied because it is sensitive to NOx, NH3, H2S, H2 and other gases.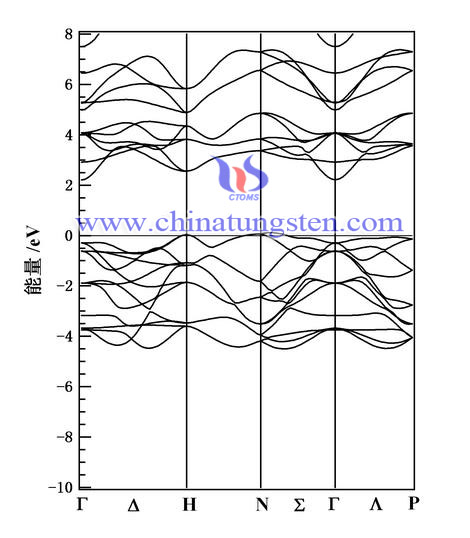
Experimental Procedure:
1. Firstly, exploring the electronic properties of doped titanium (Ti) surface WO3, establishing Ti-doped models, considering two cases of
alternative W6c Ti and W5c.
2.Secondly, studying the mechanism of Ti-WO3 sensitive surface with NO2, NH3 and H2. When Ti-WO3 surface was boiled with gas adsorption model, we take into account the four top adsorption sites: the bridge site oxygen O1c, flat bits oxygen O2c, Ti and 6 ligands tungsten W6c. NO2 and NH3 adsorption model. Bonded with N and oxygen O1c bridge of the best Ti- WO3 surface.
Conclusions:
1. The main reason for the change of resistance value is the electron transfer process by computing and analyzing of gas adsorption, density of states, and electron population in the gas adsorption, which reveals the mechanism of Ti-WO3 gas sensitive material.
2. Contrasting separately NO2, NH3 and H2 with gas sensitive mechanism and Ti-WO3, WO3 undoped, we can find that the band gap of Ti-doped causes changes in the Fermi level, the number of electron-doped model in the adsorption process is much more, the magnitude of change in resistance of the adsorption Ti-WO3 material is increasing, which will help to improve the performance ratio of WO3-based gas sensors.
3. The results of surface Ti-doped W5c has the lowest surface energy and can form a stable doping structure by calculating, the energy band structure, density of states can be obtained from analysis. The change of band gap caused by Ti doping and new electronic band, which leds WO3surface performance to change.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Method for Preparing Homogeneous Sodium Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:58
According to criterion of grain grading by Sandvik, tungsten carbide made by coarse grained [grain size 3.5~4.9μm] has advantages that good toughness, high hardness, heat conduction and red-hardness [refers to tool steel can still remain high hardness (more than 60 HRC) in the external heated ], which is widely used in the field of mining tools, die stamping, drilling for oil, hard-facing materials.
The process of conventional mechanical mixing method for preparing alkali metal doped is as follows: adding a certain amount of sodium salt into APT or yellow tungsten oxide (WO3), mixing by mixing equipment. But, because sodium salt added in mechanical mixing method is not too much, it is difficult to guarantee the uniformity of ammonium paratungstate or yellow tungsten powder, thus could easily lead to many problems like uneven granularity, incomplete crystallization, generating more fine particles and so on, and seriously affecting the properties of tungsten carbide.
In the view of the existed defects on doping sodium salt process, a simple process for preparing homogeneous sodium APT and yellow tungsten is proposed, and the preparation process is as follows:
1. Using alkali decomposition of tungsten concentrate which went through ion exchange and molybdenum removal as raw material, the concentration of WO3 required 200~300g/L, mass concentration of Na+ for 10~40 ppm;
2. Tungstate solution and compounds contain sodium mixed according the mass percent of Na/W03 for 0.05~0. 25%;
3. Evaporation and crystallization, in the temperature 80 ~ 100℃, stirring speed 50~200 r/min, pH of final crystallization point among 6.4 ~ 7;
4. Stop heating, and cooling it down to 20~50℃;
5. Filter liquid by the way of vacuum filtration, drained, drying and getting APT powder with uniform sodium, or calcined APT in the calcining furnace in 680~740℃ to generate yellow tungsten oxide powder containing uniform sodium.
The advantages of this method lie in:
1. No need to adjust the concentration of ammonia, simply production process, easy to control;
2. Low impurities content because of the high requirements of materials selection, and introducing no addition impurities, thus do not need washing products.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
The Coupling Inductance on Tungsten Trioxide of Tungsten Ores
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:55
Tungsten is metallic element. It is steel gray or silver white; it has high hardness, high melting point, and without air erosion at room temperature. The main purposes for the manufacture are the filament and high-speed cutting steel, hard mold, and for optical instruments, chemical equipment. Tungsten primarily is a hexavalent cation in nature; its ionic radius is 0.68 × 10-10m. Since W6 + ionic radius is small, the price is high, strong polarization capability, which is easy to form a complex anion, thus tungsten mainly in the complex anion form [WO4] 2-, and the solution of Fe2 +, Mn2 +, Ca2 + and other cations to form wolframite or white tungsten ore precipitation. The tungsten is a shiny silver-white metal, it has high melting point and great hardness, low vapor pressure, the evaporation rate is also smaller after smelting, and its chemical properties are more stable.
 The coupling inductance is the actual coupling coil abstract idealized circuit model; it is a linear time-invariant dual-port element, which consists of L1, L2 and M three parameters to characterize. The voltage and current relationship of the coupling inductance is the differential relation, it is a dynamic circuit elements. The coupling inductance has series in two ways - along access and reverse. A synonym, its linking is connected to terminal L1 and L2 [Fig. (A)], both from the dot end of the current i, flowing into the magnetic field in the same direction and reinforce each other. Reverse L1 and L2 is connected to the end of the same name [Fig. (B)], the current i L1 is marked from the end of the inflow from L2 that marking the end of the outflow, weakening the magnetic field in the opposite direction to each other.
The coupling inductance is the actual coupling coil abstract idealized circuit model; it is a linear time-invariant dual-port element, which consists of L1, L2 and M three parameters to characterize. The voltage and current relationship of the coupling inductance is the differential relation, it is a dynamic circuit elements. The coupling inductance has series in two ways - along access and reverse. A synonym, its linking is connected to terminal L1 and L2 [Fig. (A)], both from the dot end of the current i, flowing into the magnetic field in the same direction and reinforce each other. Reverse L1 and L2 is connected to the end of the same name [Fig. (B)], the current i L1 is marked from the end of the inflow from L2 that marking the end of the outflow, weakening the magnetic field in the opposite direction to each other.
Studying the emission spectrometry method of plasma for the determination of tungsten trioxide in tungsten ores by the coupling inductance, we select the best conditions for the instrument, in order to avoid interfering the effect on the determination, using the National Standard GBW07284 as a high standard. At a wavelength of 209.4nm, 224.8nm and 239.7nm measured tungsten trioxide, the lower limit is 0.0004% and relative standard deviation is 4.0%. Analysis by the national standard material verification method is feasible; the actual results are consistent with the analysis of samples and other analytical methods.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate from Fine Tungsten Slime
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:52
Fine tungsten slime refers to materials content tungsten hard to recover by re-election method in selecting tungsten concentrate, which the particle diameter is 0.074mm. There are three kinds of technical methods for preparing ammonium paratungstate (APT) by using fine tungsten slime as following: classical, alkali extraction-ion exchange and alkali-extraction method.
Processing of low grade wolfram disposed with classical method is like below: alkaline leaching→ sodium tungstate solution crystallization→ P, As, Si removal→ molybdenum removal→ hydrochloric acid decomposition→ dissolution with ammonia→ ammonium paratungstate crystallization. The advantages of classical method are mature technology, simple equipment, easy to operate. However, the shortages are longer process, strict requirements of impurity content in material; besides, two specialized processes for impurities removal are needed.
A new process for preparing APT from fine tungsten slime is based in the classic method, and using appropriate technical measure, controlling the right conditions, simplify removing impurity process, save two steps in the classical method. Concrete steps are as following: alkali leaching→ sodium tungstate crystallization→ stirring and lye→ decomposition with hydrochloric acid→ ammonia solution dissolution with ammonia→ ammonium paratungstate crystallization→ stirring and washing in NH4Cl solution.

The advantages of this method are as follows:
1. Solve the problems separated W and impurities like Mo, P, Si, As effectively, do not need a special purification process, so as to shorten the process, less equipment investment, reducing production costs, and with significant economic benefits;
2. Tungsten smelting total recovery rate can reach 90% or more, and APT with high purity and grain that can be controlled can be directly prepared reference to WO3GB3457-82;
3. The scale of production is not limited, can be large or small, strong adaptability to raw materials;
4. Simple equipment, extensive auxiliary material source, easy to operate;
5. To the environmental benefits, less displacement of waste gas, water and solid, and meeting industrial emission standards only simple handling.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Impurity Sodium in Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:45
Ammonium paratungstate (APT) is an important intermediate product in tungsten metallurgical process, the characteristics and quality of the final tungsten products directly affect by impurities in APT, and the size of tungsten powder related to content of sodium (Na) . Ammonium paratungstate with high sodium calcined to produce WO3 powder with high sodium which is the raw material for preparing coarse tungsten powder. On the contrary, tungsten powder with fine particles generated fromAPT with low sodium. Therefore,for producing fine particles of tungsten powder, the necessary to control content of sodium in APT in a lower state is self-evident.

Sodium ions (Na+) exists in APT crystallization solution mainly in the form of sodium tungstate, and because of the similarity in composition and structure between APT and sodium paratungstate, sodium may enter APT lattice in the form of alternative or impurity ions or surface chemical adsorption. There are two sources of impurity sodium in ammonium paratungstate:
1. The water
Using single stage double bed purification process, the average electrical resistance of pure water can controlled in 8*104Ω*cm, and no impact on the quality of tungsten products because of the low sodium in water.
2. The ammonium chloride in the desorption agent
The content of sodium is really large in APT produced ion exchange method, generally reach (3-7) *10-2%, far more than the highest value-0.01 [According to fine tungsten powder metal elements analysis table], and therefore will affect the fine tungsten powder. In this case, the sodium content not only related to its concentration, but also factors of over-saturation, nucleation rate, crystal growth rate, size of generated crystal, morphology and surface and solution viscosity and so on, and these factors listed are influenced by the concentration of WO3.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Effect of Powder Property on Tungsten Copper Electrode Density (2 of 2)
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:40
Relevant foreign scholars have studied the densification process high-density tungsten alloy found W-Ni-Fe high density alloys, when the average particle size of the tungsten particles is 1μm, occurred at about 1200 ℃ rapid densification; when the tungsten particles have an average particle size of 5μm, at 1400 ℃ was undergoing rapid densification. It indicates that decreasing the granularity of powder and increasing the superficial area can remarkably decrease the sintering temperature of powder press.
In addition, the appearance of powder particles has an influence of densification processing. If the shape of the powder particles exhibit uneven shape it is easy to bypass the formation of voids between the particles and increases the friction powder and die wall, between the powder particles, which will not help to improve the sintered density after the bulk material. The higher the degree of spherical particles, the better mobility, it is easily to fill the cavity so that bulk density, but also conducive to pressing and sintering, to obtain a higher density. Nano tungsten powder and nano copper powder SEM image as follow:
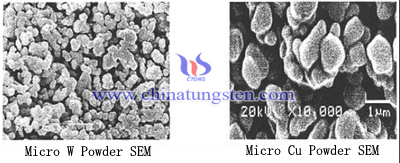
Viewed from the SEM image, we can find that micro Cu powder particle has more regular shape and high sphericity; compared with micro Cu powder, micro W powder particle is more irregular, it is polygonal and has low sphericity, which is not beneficial for tungsten copper electrode with high density manufacturing. Furthermore, micron tungsten powder and copper powder having a large surface area and the ratio of the excess surface energy, in a state of energy imbalance, has a high activity, favor the sintering process. Cu particles has good plasticity and soft texture, easily deformed during pressing and help to increase the contact area between the particles so that the tungsten copper electrode material density may be improved; however, W particles has high melting point, high hardness, low plasticity, it is not easily to be out of shape and fractured, which is not beneficial for the density improving.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Multi-Dimensional Network Structure Tungsten Trioxide Preparation
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:38
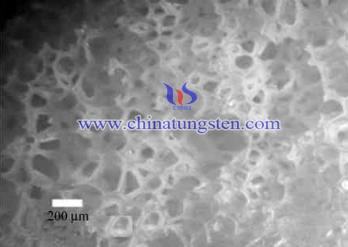 At present, people have been able to prepare different pore sizes of porous tungsten oxide and have been prepared using a variety of methods and materials that containing a large hole, mesoporous and microporous, or only two of the pore size. These materials have important applications in catalysis, selective separation and sensors; however, the porous material with a foam shape is generally more fragile and friable. Tungsten trioxide is a transition metal oxide that is widely studied because of its unique properties. In addition, the doping tungsten trioxide complex also has a catalytic effect. It means that tungsten trioxide morphology and structure of the desired application function has a direct relationship.
At present, people have been able to prepare different pore sizes of porous tungsten oxide and have been prepared using a variety of methods and materials that containing a large hole, mesoporous and microporous, or only two of the pore size. These materials have important applications in catalysis, selective separation and sensors; however, the porous material with a foam shape is generally more fragile and friable. Tungsten trioxide is a transition metal oxide that is widely studied because of its unique properties. In addition, the doping tungsten trioxide complex also has a catalytic effect. It means that tungsten trioxide morphology and structure of the desired application function has a direct relationship.
Steps:
Preparing dimensional network structure WO3 containing mesopores by concentrating hydrogen peroxide (H2O2), methanol, over-acid and polyvinylpyrrolidone (PVP) solution. Preparing WO3 mesh structure by using optical microscopy, scanning electron microscopy (SEM), powder X- ray diffraction (XRD), thermal gravimetric analysis (TG), high-resolution transmission electron microscope (HRTEM) and N2 adsorption isotherms (BET) technology after firing.
Conclusions:
1. Optical microscopy and SEM photograph show this shape of the foam cube mesh structure regardless of WO3 is relatively stable before calcination or after calcination, supported by the nature of the performance.
2. Magnified SEM image shows that the wall mesh structure is assembled from nanoparticles made of WO3 after calcination.
3. XRD diffraction analysis shows that WO3 is amorphous foam before calcination, and is orthorhombic crystals after calcination. As PVP structure, it has the advantage of inducing inexpensive agents and stable solution.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
New Types, Structure of Coated Cemented Carbide—Diamond Coating 2 of 3
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 17:37
Take YG6 tungsten carbide drawing die as an example, it deposits diamond coating (10-30pm crystalline diamond coating) on tungsten carbide drawing die matrix by chemical vapor deposition (CVD) and the finished products after polishing, finishing and some after processing. In general, the processes of tungsten carbide drawing die with diamond coating are tungsten carbide matrix milling → matrix washing → pre-processing → pretreatment → put in furnace → diamond coating deposited → film testing → finishing and polishing → finished product.
First of all, choose YG6 tungsten carbide die (the shape and size should be similar to the finished product). Then bore milling and finishing for proper shape, and reserve the size margin about 30μm to cooperate with the thickness of the coating layer. Afterwards, it adapts sand blast to eliminate the impurities and loose material of the surface, which can achieve good roughness. After sand blasting, it should be ultrasonic cleaning by distilled water and alcohol. Finally, the drawing die corroded by the acid or alkali prepared and ultrasonic cleaning by distilled water and alcohol again. When the preparation complete, put tungsten carbide drawing die into the equipment of CVD diamond coating deposited.
The most common equipment of coating deposited is hot wire furnace, which is equivalent to a reaction chamber. the gas containing other elements constituting the respective film gaseous reactant vapor or liquid reactants and reaction necessary introduced into the reaction chamber. That is, after the pre-pass hydrogen H2 and methane, CH4, hot filament heated to 2500 ℃, by adjusting the filament temperature, gas pressure, gas flow rate and relevant process parameters, so that the mold surface of diamond film deposition of a certain thickness. There are special requirements for accuracy or surface finish can be achieved by further optimizing the surface quality of the finishing and polishing process precision, to meet different working environment for the corresponding requirements of the mold.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Roughing in Wolframite Beneficiation Processing
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 16:48

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Wolframite Beneficiation Processing
- Details
- Category: Tungsten Information
- Published on Monday, 11 January 2016 16:40

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com