Nano Silver Tungsten Trioxide Preparation
- Details
- Category: Tungsten Information
- Published on Friday, 08 January 2016 16:55
There are many problems in traditional sewage treatment methods, such as adsorption, coagulation, activated sludge methods which are low efficiency, high cost, no secondary in pollution. In the grim background of global energy shortage, environmental pollution problems have become increasingly. The efficient use of clean, no secondary pollution photocatalytic technology of solar energy is one of the preferred methods of treatment of pollutants. Taking tungsten trioxide and nano-materials as the matrix, nano-silver tungsten trioxide is commonly used in the preparation of photocatalytic degradation of organic pollutants and photolysis of water. This method belongs to the field of inorganic photocatalyst preparation technology, its catalytic efficiency can be greatly improved compared with other methods of, and the process is simple, environmentally friendly.
 Experimental Method
Experimental Method
Taking tungsten trioxide and nanometer chip as the matrix, by UV reduction method, making nano-silver uniform load on the tungsten trioxide nanosheets, and then making it be baked under certain temperature in a wet state, which can obtain the new photocatalyst nano-silver loading tungsten trioxide so as to achieve the purpose of improving the tungsten oxide photocatalytic activity. Testing samples with nitrogen (N2) adsorption - desorption, X-ray diffraction (XRD), transmission electron microscopy (TEM), diffuse reflectance spectroscopy (DRS) and photocurrent.
Results
The catalyst of tungsten trioxide photocatalyst supported nano silver with intact crystalline and regular pore structure is conducive to light green separation of electrons and holes. Meanwhile, as nano-silver tungsten trioxide solid state electronic receptor and transport the body to promote the photo-generated electron - whole pairs of transport and separation, this effectively improves the visible light catalytic performance. Doped with 2% (mass fraction) composite photocatalyst performance nano-silver tungsten trioxide is best, 4h 4-CP degradation can be more than 96%, catalyst can be reused many times, and its photocatalytic activity remained unchanged.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
High Purity Tungsten Trioxide Preparation
- Details
- Category: Tungsten Information
- Published on Friday, 08 January 2016 16:39
The resources of China's tungsten ore are very rich, however, the reserves and production of high grade, easy exploitation of black, white tungsten ore declining sharply because of years of exploitation. So far, high-purity tungsten is also largely depended on imports. High-purity tungsten trioxide powders are indispensable raw materials in the electronics industry .It has been widely used in magnetron sputtering and large-scale integrated circuits. It can be used as a raw material made of metal tungsten, carbide manufacturing, cutting tools, molds and pull tungsten wire, it can also be used for powder metallurgy, it can be used for X-ray screens and fireproof fabrics, as well as coloring agents. Therefore, the preparation of high purity tungsten trioxide becomes an important research.
The Experimental Method:
The raw materials of tungsten trioxide are based on superior grade ammonium (APT) by calcination processes. The outputs of the original high-purity tungsten powder, including molybdenum 40mg / kg, iron 20mg / kg, about 95% tungsten powder. The preparation of high concentrations of hydrochloric acid and hydrogen peroxide is based on nitric acid ammonium paratungstate, which can produce high-purity tungsten powder, including molybdenum that containing 4mg / kg, iron 1mg / kg, tungsten ≥99%.
The Experimental Results:
1. When the solution that adding WHO3 was boiled in the experiment, there are a large amount of bubbles during the precipitation, which plays the role of loosing tungstate, eliminating the artificial stirring, which is more obvious than plusing H2O2.
2. The precipitation of tungstate from paratungstate in high acidity (HCl> 8 mol / L), which can significantly reduce the quality of hetero-containing.
3. In the hydrolysis process of paratungstate acid, adding hydrogen peroxide (H2O2 ,100ml/kg) and nitric acid (the amount of hydrochloric acid 1/10), which can reduce the impurity content.
4. There is a difference of dissolve between a large number of H2NO4 and a very small amount of H2MO4, and H2WO4 is completely precipitated, H2MO4 is dissolved in the high acidity (hydrochloric acid or nitric acid), which achieves separation.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sulfur- Tungsten Trioxide Detection
- Details
- Category: Tungsten Information
- Published on Friday, 08 January 2016 16:19
N Carbide and alloy performance is largely determined by the quality of tungsten trioxide. Common methods of preparing tungsten trioxide including the following major steps: first of all, removing sulfur, silicon, fluorine, arsenic, phosphorus, and molybdenum by solution purification, secondly, the precipitation of artificial scheelite based on calcium chloride solution. Thirdly, using hydrochloric acid to decompose artificial scheelite; finally, the tungsten trioxide is prepared by washed, filtered, dried and calcined in tungstate. This article focuses on the methods for determination of sulfur in high-purity tungsten trioxide.
 The definition of Colorimetry is based on the color reaction of the colored compound by measuring or comparing the depth of the colored substance solution. As a method of quantitative analysis, the basic requirements of the Colorimetry are: high sensitivity and selectivity, stable colored compound and different color. The color reaction and control of the reaction conditions is the key for colorimetric analysis.
The definition of Colorimetry is based on the color reaction of the colored compound by measuring or comparing the depth of the colored substance solution. As a method of quantitative analysis, the basic requirements of the Colorimetry are: high sensitivity and selectivity, stable colored compound and different color. The color reaction and control of the reaction conditions is the key for colorimetric analysis.
Determination of Sulfur in High-Purity Tungsten Trioxide Method
Burning the fat high-purity tungsten trioxide at 1400 ℃, and the resultant of sulfur sulfide reacts with oxygen form sulfur dioxide. This compound is condensed with formaldehyde, and the purple compound can generate in 560nm colorimetric determination. The experiment of the color, the time and the sample of absorption time can reduce combustion conditions such as blank test. Finally, we can obtain the amount of available mercuric chloride, sodium, chromogenic agent, the influence of magenta and fading. Detection limit of this method is 0.5ppm; the relative standard deviation is ± 4.8%.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Ore Associated Resources Comprehensive Recycling Utilization
- Details
- Category: Tungsten Information
- Published on Friday, 08 January 2016 14:35

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Ore Mining in China
- Details
- Category: Tungsten Information
- Published on Friday, 08 January 2016 14:28

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonia Condensation in Ammonium Paratungstate Crystallization Affecting Factors
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 20:20
Condensation of ammonia tail gas from ammonium paratungstate is affected by many factors. Condensing often refers to the physical condensation process of the constant or high temperature gas, liquid cooled down, also reaction produced by polymerization of two or more physical. Studying the law NH3 saluted in water t different temperatures, and adopting appropriate technology and equipment will make a good effect on NH3 recovery. We analyze from the following 3 factors:
1. Area ratio of condensation and evaporation (Short for Sc/Se)
The concentration of ammonia decreased with the increasing of Sc/Se in APT crystallization. In the early evaporation stage, low temperature of the solution, high concentration of ammonia, much NH3 escaped and small amount of evaporation lead to higher concentration of ammonia; in the latter stages, temperature rising lead to ammonia concentration reduced, and less NH3 escaped, moisture evaporation, condensation of ammonia declining. When in the right Condensation strength, NH3 absorbed in water at maximum; the smaller Sc/Se is disadvantage to absorb ammonia gas; the larger Sc/Se will increase the condensation amount, resulting in the decrease of the concentration of ammonia.
2. Evaporation temperature
The saturation water vapor will increased with the evaporation temperature increasing, and the more water condensation will dilute ammonia, so low temperature evaporation is conducive to improve the concentration of ammonia.
3. Stirring speed
Evaporation of H2O and evaporation are the process of liquid→gas during the APT crystallization, and stirring can promote the concentration of ammonia.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate -Hydrogen Peroxide System Catalyzing-Degrading Methylene Blue
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 19:55

| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate and SLS Composite Corrosion Inhibition to Carbon Steel 2/2
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 19:48
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate and SLS Composite Corrosion Inhibition to Carbon Steel1/2
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 19:46

| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Indirect Condensation Absorption Disposing Ammonia in Ammonium Paratungstate Crystallization
- Details
- Category: Tungsten Information
- Published on Thursday, 07 January 2016 18:20
Ammonium paratungstate (APT) crystallization is the process of the polymerization of ammonia and acid. Ammonia (NH3) volatiles out from the solution the volatilized ammonia in the heating process. It will cause atmospheric pollution if ammonia discharged directly into the air. Ammonia combines with hemoglobin easily and destroys the function of oxygen transportation, even lead to death.
Ammonia, commonly known as NH3, is a colorless and tasteless gas, with a strong pungent odor, easily soluble in water, 1 volume of water can be dissolved 700 times the volume of ammonia under normal temperature and pressure. The environment department of China stipulates that waste water of tungsten smelting is the first class of elements to discharge the waste gas, zero discharge requirements is demanded. So, it is necessary for enterprises to take a new and efficient way of recycling tail gas in APT crystallization, in order to achieve the goal of zero emissions of ammonia gas.
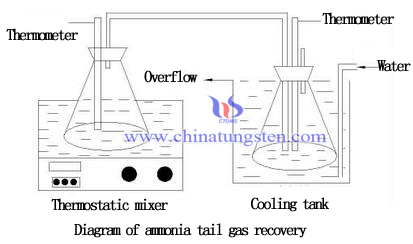
Indirect condensation method is an effective method for recovery of ammonia from APT tail gas. Two ways to recover ammonia produced from ammonium paratungstate crystallization.
1. Condensed into ammonia and returned to the reaction process;
2. Converted into NH4Cl and return to desorption of ion exchange process.
Compared the two ways, the front is of higher economic value. NH3 and water are polar molecules, ammonia and water molecules combining and becoming a hydrogen bond when NH3 soluble in water. With temperature increasing, the solubility of ammonia in water becoming lower, so the low-temperature condensation is an effective way to improve the recovery rate of ammonia.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com