Preparing Sodium Tungstate from APT Crystal Mother Liquor
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 20:50
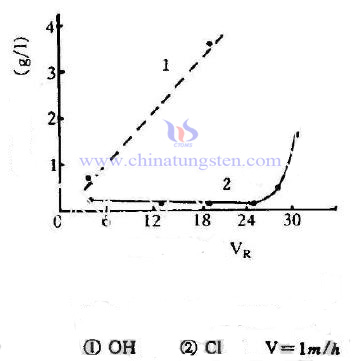 Using ion exchange method can recycle sodium tungstate from APT crystal mother liquor. Through the experiment, following conclusions can be drawn:
Using ion exchange method can recycle sodium tungstate from APT crystal mother liquor. Through the experiment, following conclusions can be drawn:| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ion Exchange Method Recycling Sodium Tungstate from APT Crystal Mother Liquor
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 20:44
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate Solution Activity Coefficient
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 20:41
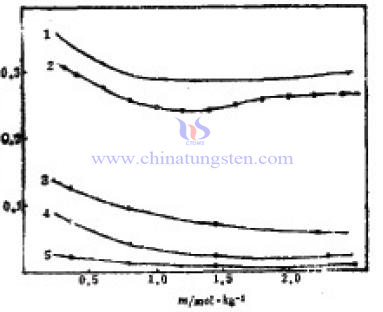 Due to the different measuring methods of sodium tungstate in the aqueous solution activity coefficient, the results have larger differences. This paper combining with inorganic salt aqueous solution thermodynamic properties, measures the activity coefficient of sodium tungstate in aqueous solution by the equal-pressure method. Experimental methods can be divided into following three points:
Due to the different measuring methods of sodium tungstate in the aqueous solution activity coefficient, the results have larger differences. This paper combining with inorganic salt aqueous solution thermodynamic properties, measures the activity coefficient of sodium tungstate in aqueous solution by the equal-pressure method. Experimental methods can be divided into following three points:| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CVD Diamond Coated Carbide Cutting Tool——Technical Matters (2/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 18:19
Last but not the least, the binding force or adhesion is the most important problem of diamond coating and tungsten carbide matrix, which is also the major technical matter of most of coated cutting tool confronted with. Theoretically, in the process of machining, if the adhesive force between diamond coating and the matrix is too low, CVD diamond coating will be peeled off and become invalid early under the collective effect of the cutting force and the friction (especially in the cutting process with high speed or high load), which largely decreases the cutting property and service life of coated cemented carbide cutting tool.
However, the most difficult problem of the binding force confronted with is cobalt (Co) in tungsten carbide. Due to Co will promote the conversion of graphite to diamond (graphite and diamond are allotrope, and Co can be synthesized to the catalytic of diamond under high pressure) under high temperature and high pressure. But it will promote the growth of graphite under low temperature and low pressure (the growth conditions of CVD diamond).
Thus, CVD diamond is hard to nucleate and it has a huge influence on the binding force of diamond film and becomes invalid. In order to improve the binding force of CVD diamond coating and tungsten carbide matrix, we should take advantage of pre-treatment. The related researchers has proposed some new process, such as acid erosion or plasma etching to remove cobalt (Co), applying a variety of over-plating, mechanical or chemical heat treatment and so on. Although these methods are still in a development stage, it indicates that CVD coated cemented carbide cutting tool has a broad application prospect.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CVD Diamond Coated Carbide Cutting Tool——Technical Matters (1/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 18:14
Based on tungsten carbide cutting tool, it deposited a layer of diamond coating by using chemical vapor deposition (CVD), which makes it not only have the properties of tungsten carbide, but also have the excellent properties of diamond coating, such as high hardness and good wear resistance. Evaluation of the quality of diamond coating is mainly in the structure of film, the defects, especially the existence of the cracks. The existence and expanding of the cracks will remarkably decrease the comprehensive properties and serve life of the cutting tools. Therefore, in order to optimize diamond coated cemented carbide cutting tool, some new processing are emerging based on traditional chemical vapor deposition (CVD) and physical vapor deposition (PVD), such as low pressure CVD, plasma CVD, vacuum cathodic arc deposition (VCAD), DC arc plasma spraying CVD, hot wire CVD and so on.
Next, sharpening process after finishing is also the major problem in diamond coated cemented carbide cutting tool. Due to the diamond coating by CVD is composed of coarse particles, which is difficult to ensure high precision cutting. In addition, sharpening process is too complicate to have a bad influence on the quality and service life of diamond coating during the process. Thus, the relevant researchers through improved diamond film deposition process conditions to obtain micron, even nano diamond film, which makes the sharpening process not so necessary and decrease the fabricating cost and effectively avoid the problems of sharpening process brings in.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Polymorphic Nanosize Tungsten Trioxide Produced Continuously from Ammonium Paratungstate 2/2
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 17:19
Equipment requirements: fused quartz tube with air inlet and outlet pipe; tubing furnace; ceramic crucible.
Reactants: carbon cloth; ammonium paratungstate (APT); argon.
Specific steps as following:
1. Take out fused quartz tube, place carbon cloth at the end of inner wall of air outlet, and then sent fused quartz tube with carbon cloth traverse in a tube furnace;
2. Heating tube furnace to make sure the fused quartz tube heating up to the temperature of 1250°C-1400°C, and the heating rate keeps in 50°C/min;
3. Sent the ceramic crucible with APT into the used quartz tube, and keep it in zone of specified temperature, blowing argon with flow lL/min-6L/min;
4. Keep the temperature in the specified value for 40~60min, turn off the inlet and stop blowing argon;
5. Take out carbon cloth to collect polymorphic nanosize tungsten trioxide, and remove out the ceramic crucible;
6. Repeat steps 1-5, to achieve continuous production.
Noted that work as following should be done at each cycle:
1. Clean the fused of quartz tube for removing residual products, if the wall isn’t washed, the contamination of subsequent preparation will be occurred;
2. Place a new carbon cloth around the inner wall of the glass tube;
3. Take the ceramic crucible with APT to temperature constant zone;
4. Inlet argon again.
The advantage of this method is that the size and purity of WO3 powder can be controlled because of the high purity APT selected; preparation process simplified, shorten production cycle; lower cost and energy consumption. At the same time, the loose degree of the powder generated is really high, and there is no need to broken again.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Polymorphic Nanosize Tungsten Trioxide Produced Continuously from Ammonium Paratungstate 1/2
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 17:17
Tungsten trioxide (WO3) is a kind of importantηtype semiconductor oxides with varieties of crystal structure, and has been applied in fields of electrochromic, photochromic, sensing, and catalysis. The size of quasi-spherical and spherical tungsten trioxide is respectively 100nm-700nm and 20-200nm. These two kinds of WO3 can used as precursor for synthesing nanosize tungsten carbide; the length of diagonal of octahedral tungsten trioxide is less than 1μm, used as gas sensing to produce gas sensor because of the high sensitive; the length of diagonal of WO3 with irregular polyhedral angle is about 10nm-2μm, and it can be used to adsorb organic molecules, or used to photodegradate organic material. The more fine particles the WO3 is, the more superior property the subsequent products. Therefore, reducing size of WO3 is the best way to further utilize the excellent properties of WO3 and broaden its application field.

Vapor Deposition Method is one of the most effective methods to prepare nano structure, and a variety forms of particles produced by the technology of vapor deposition, such as thin films, whiskers and particles ect.. And the Vapor Deposition Method can produce product with high purity from the low concentration of reactants. But the disadvantage of conventional Vapor Deposition Method is the limited yield; and it can’t achieve producing continuously because of products collected in the environment of high vacuum or temperature. This paper presents a method that can produce polymorphic nanosize tungsten trioxide continuously, take APT.XH2O as raw material, innovatively combine Vapor Deposition Method with calcination, take argon as carrier gas, collect the products in the area of low temperature, and then achieve the goal of producing polymorphic nanosize tungsten trioxide continuously.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nanosize Tungsten Trioxide Produced by Electro-Pyrolytic from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 17:13
Tungsten trioxide (WO3) is yellow powder, a kind of important tungsten oxide, also known as yellow tungsten oxide. At present, it’s mainly used in the manufacture of tungsten powder (W) and tungsten carbide powder (WC). The main final products of WO3 are tungsten rod, tungsten wire and tungsten carbide and so on. In order to obtain WO3 with better chemical activity, and meet the demand of special alloy with superior properties, we propose a new method--Electro-Pyrolytic to prepare nanosize tungsten trioxide.

Equipment: electromagnetic stirrer; evaporator; drying equipment; grinding equipment; crucible.
Reaction materials: high purity of ammonium paratungstate (APT) with size about 5 μm, purity greater than 99.9%; distilled water; citric acid; ammonia; oxygen.
Specific steps as follows:
1. Adding distilled water into the reaction vessel, and use electromagnetic stirrer for mixing, adding citric acid solution with stirring;
2. When the citric acid solution and distilled water are mixed evenly, adding high purity of APT with stirring, the molar ratio of APT and citric acid is 1:3;
3. Adding ammonia with concentration of 30% to APT solution, stirring at the speed of 180-200r/min;
4. Place the reaction solution in the evaporator to concentrate, drying the concentrated liquid in the drying equipment at the temperature of 150~160°C, and then get the precursor;
5. Precursor grinded in the grinding equipment, heating it in a crucible with oxygen, finally gets nanosize tungsten trioxide. Calculate the flow of oxygen at each gram of precursor for 50-60ml/min, heating temperature for 500~520 °C, time for 1.2~1.5h.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Wolframite and Tungsten Ore
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 17:07

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Spectrophotometry Determine Fe Element in Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 January 2016 17:06
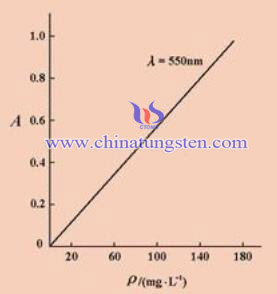 Spectrophotometry is qualitative and quantitative analysis method for the substance by measuring the absorbance or emission intensity of the test substance in a specific wavelength or range of wavelengths of light. When the solution is a bunch of intensity I0 monochromatic vertical irradiation of a substance, due to the portion of the light is absorbed by the system, thus the intensity of the transmitted light is reduced to I, T is the light transmittance of the solution is: according to Lambert (Lambert) - Beer (Beer) Law: A = abc. Where A is the absorbance, b is the thickness of the solution layer (cm), c is the concentration of the solution (g / dm ^ 3), a is the extinction coefficient. There are relationships between the absorption coefficient of the nature of the solution, temperature and wavelength and other factors. Solutions of other components (solvents, etc.) are available to deduct by blank liquid absorption of light ion.
Spectrophotometry is qualitative and quantitative analysis method for the substance by measuring the absorbance or emission intensity of the test substance in a specific wavelength or range of wavelengths of light. When the solution is a bunch of intensity I0 monochromatic vertical irradiation of a substance, due to the portion of the light is absorbed by the system, thus the intensity of the transmitted light is reduced to I, T is the light transmittance of the solution is: according to Lambert (Lambert) - Beer (Beer) Law: A = abc. Where A is the absorbance, b is the thickness of the solution layer (cm), c is the concentration of the solution (g / dm ^ 3), a is the extinction coefficient. There are relationships between the absorption coefficient of the nature of the solution, temperature and wavelength and other factors. Solutions of other components (solvents, etc.) are available to deduct by blank liquid absorption of light ion.
From the above equation, when the thickness of the solution layer b and a are fixed a, which can show a linear relationship in the concentration of the solution and the absorbance A. When we have quantitative analysis, determine a solution for absorption of different wavelengths of light (absorption spectrum) is needed, which determines the maximum absorption wavelength. We take this wavelength of light as the light source, a series of known concentrations measured absorbance c solution A, and making A ~ c curve. In the analysis of an unknown solution that is based on the measured absorbance A, checking the working curve we can determine the corresponding concentrations. This is the basic principle of spectrophotometry measurement of concentration.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
We find the stability enables the method sensitivity, contrast and enhanced complex improving in the test of 5-Br-PADAP-Pe (Ⅱ) system that adding non-ionic surfactant emulsifier OP and ethanol. In the conditions of 5-Br-PADAP-Fe (Ⅱ) complex formation emulsifier OP pluralism, and in the presence of ethanol and an emulsifier OP, pH3.5-10H Ac-NaAc buffer medium, Fe (Ⅱ) with 5-Br -PADAP generates purple complex at a wavelength of 558nm, the Fe content in the 0-60μg / 50ml law is obeyed in the range, the complex apparent molar absorption coefficient is 7.64 × 10 ~ 4. Color remains completely within five minutes, the absorbance is stable within 24 hours at room temperature, this method has good selectivity.



 sales@chinatungsten.com
sales@chinatungsten.com