Nanosize Cesium Tungstate Powder Prepared from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 20:13
Nanosize cesium tungstate powder is widely used in field of transparent insulation thin film such as EVA film, optical filter and so on. And it can also used in laser marking and welding, near infrared light heat treatment drugs.
Cesium tungstate has unique properties in different bands of light as follows:
1. The absorption property is very strong in the near infrared region of the wavelength 800-1100nm;
2. The property of light penetration is so obvious in the visible light region with the wavelength of 380-780nm;
3. Shielding property is very strong in UV light region at the wavelength of 200-380nm.
In order to reduce the preparation cost and the degree of agglomeration, simplify the process and improve the production efficiency, so that nanosize cesium tungstate powder can be more widely used. A new method for preparing nanosize cesium tungstate powder by using ammonium paratungstate, cesium carbonate and methanol as raw materials is proposed.
Specific steps are as follows:
1. Dissolving ammonium paratungstate and cesium carbonate in methanol solution, drying to get powder;
2. Crushing the powder particles obtained from step 1 to obtain tungsten and cesium powders mixed;
3. Placing the powder obtain in step 2 in a sealed reactor with stirring, adding sorbitol, then heated to 350℃ and stirring for 2~8 hours;
4. Separate the products produced in step 3, and washing with alcohol, then drying, and crushing in the air flow mill to get nanosize cesium tungstate powder.
Remark: The air pressure in the crushing chamber is 0.6MPa~l.IMPa, and the rotor speed is set to 1000 revolutions per minute.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate and Dioxopromethazine Hydrochloride Influence Factors
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 19:50
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate and Dioxopromethazine Hydrochloride Spectral Properties
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 19:44
 Light scattering phenomenon is so widespread in light and the particle in the process. It is refers when a beam of light passes the medium in the direction of the incident light in all directions outside, the phenomenon of a kind of optical radiation can be observed. Light scattering phenomenon is widespread in nature. Resonance light scattering technique using resonance light scattering probe mainly organic reagents, inorganic ion probe is less reported. In this paper, based on in phosphoric acid medium, the protonation of dioxopromethazine hydrochloride can form with polycondensation of tungsten acid radical ion association. The system resonance light scattering intensity is enhanced. And the intensity and the concentration of dioxopromethazine hydrochloride are within a certain scope to establish a new method for determination of dioxopromethazine hydrochloride. It’s used in testing dioxopromethazine hydrochloride in drgus.
Light scattering phenomenon is so widespread in light and the particle in the process. It is refers when a beam of light passes the medium in the direction of the incident light in all directions outside, the phenomenon of a kind of optical radiation can be observed. Light scattering phenomenon is widespread in nature. Resonance light scattering technique using resonance light scattering probe mainly organic reagents, inorganic ion probe is less reported. In this paper, based on in phosphoric acid medium, the protonation of dioxopromethazine hydrochloride can form with polycondensation of tungsten acid radical ion association. The system resonance light scattering intensity is enhanced. And the intensity and the concentration of dioxopromethazine hydrochloride are within a certain scope to establish a new method for determination of dioxopromethazine hydrochloride. It’s used in testing dioxopromethazine hydrochloride in drgus.| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CVD Diamond Coated Carbide Cutting Tool Pretreatment—Plasma Etching
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 18:06
Plasma etching is one of processing that can be applied in most of matrix, which including plasma cleaning, plasma activation, plasma coating for some complicate structure. It can not only remove cobalt (Co), but also has decarburization when it used in CVD diamond coated carbide cutting tool, which uses H, O, Ar atom or ion and CO in H2, O2-H2, H2O-H2, CO-H2, Ar-H2 to react with tungsten carbide (WC) and binder phase cobalt (Co) forms CO2, CH4, Co(CO)4, Co(OH)4 and other compounds and Co hydrates with highly volatile. So pure tungsten carbide surface to form a layer of a certain thickness, beginning after the deposition of the diamond layer is carbonized again produce a chemical bond and form new tungsten carbide WC particle.
As a result, in the early stage of diamond deposition, CVD diamond coating between the substrate and the formation of carbide cutting tools tungsten carbide (WC)intermediate layer. It not only can effectively reduce the residual stresses present in the film, but also to some extent, but the barrier of Matrix diamond growth had spread to the deep cobalt heavy surface, and the diamond crystals embedded into the WC grain boundaries, so that the diamond the contact area between the film and the cemented carbide substrate is improved, resulting in "pinning" effect, effectively improving the inter-layer film with the adhesion between the film and the substrate. Plasma etching is a form of dry etching, the principle is exposed to the gas to form a plasma electron region, electricity gas and the resulting release of gas energy electrons to form a plasma or ion, electricity gas when atoms accelerated by an electric field, it will release sufficient strength and surface forces expelled only adhesive material or etched surface. In addition, plasma etching can be specifically divided into reactive plasma (RIE), downstream plasma, direct plasma and so on.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Spectrophotometry Determines Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 17:44
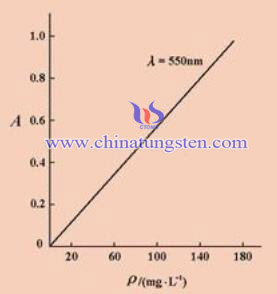 Spectrophotometry is a method of ualitative and quantitative analysis of testing substance the absorbance or emission intensity of in a specific wavelength or range of wavelengths of light .In the quantitative analysis, the first need to a solution for absorption is different wavelengths of light (absorption spectrum), which determines the maximum absorption wavelength, then this wavelength of light can be as the light source, a series of known concentrations measured absorbance c solution A, making A ~ B curve. Due to the large number of new synthetic coloring agents, making the element sensitivity of the assay in the progress, especially applied research related to multicomponent complex and various surface active agents, in which many molar extinction coefficients of elements from the original raises to tens of thousands to hundreds of thousands.
Spectrophotometry is a method of ualitative and quantitative analysis of testing substance the absorbance or emission intensity of in a specific wavelength or range of wavelengths of light .In the quantitative analysis, the first need to a solution for absorption is different wavelengths of light (absorption spectrum), which determines the maximum absorption wavelength, then this wavelength of light can be as the light source, a series of known concentrations measured absorbance c solution A, making A ~ B curve. Due to the large number of new synthetic coloring agents, making the element sensitivity of the assay in the progress, especially applied research related to multicomponent complex and various surface active agents, in which many molar extinction coefficients of elements from the original raises to tens of thousands to hundreds of thousands.
Spectrophotometric method is widely used in the detection of tungsten trioxide in geological rock minerals. In the experiment, the spectrophotometric instrument is generally 721. Reagents normally are solid sodium peroxide, sodium hydroxide, hydrochloric acid solution. In hydrochloric acid (HCL) medium, using thiocyanate as the reducing agent, putting it in a high temperature of about 700 ℃ for melting,, and after cooling into 250ml beaker of hot water, after boiling and cooling, diluting into 100ml bottles, mixing to a certain scale. 20g of sodium hydroxide solution is added to HCL, after adding 4ml250g / LKSCN solution continue mixing and shaking, then adding 20ml hydrochloric acid, diluting with water to a certain moment, after ten minutes, we can have absorbance measurements. By spectrophotometry, generating a stable yellow-green complex with a tungsten content of the solution is clear reference solution; the absorbance of the solution is measured at a wavelength of 400nm.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Electrode and Rare Earth Tungsten Electrode
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 17:35

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Electrode
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 17:32

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Asynchronous flotation of Scheelite and Wolframite Mixture Ore
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 17:31

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Detinning Process of APT Production- Additives in Alkali Decomposition
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 17:28

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Recycling Tungsten Trioxide in Waste SCR Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 17:23
The development of SCR denitration catalyst is based on the technology of selective catalytic reduction (SCR), the SCR system is an integral and important part of SCR systems. In performance, the denitration catalyst is the key to success of the project, its components, structure, life and its associated parameters directly affect the health of denitration efficiency and SCR systems. SCR denitration catalyst can be classified according to different raw materials, structure, extent, use and other standards. There are mainly three types in SCR denitration catalyst: 1) precious metal type; 2), metal oxide type; 3), zeolite type of ion exchange. The type of metal oxide is currently the more popular, it is also more widely used, and such catalysts is based on the carrier of titanium dioxide, vanadium pentoxide, tungsten trioxide the active ingredient.
Steps
(1)Crushing SCR denitration catalyst to dry powder, and roasting it in high-temperature;
(2) Adding a solution of soluble tungsten trioxide component and heating it in the above step (1) that the resulting is dry powder;
(3) Collecting the supernatant after fully dissolving, and make the liquid-solid layers clean;
(4) Adding the solid in the step (3) and the composition of tungsten trioxide to the reaction vessel together, and repeating the step (2);
(5) Repeating the step(3) and (4) at least once;
(6) Collecting several components and dissolving tungsten trioxide that was evaporated to dryness and sufficiently, drying to obtain a solid;
(7) Sintering the resulting solid in the step (6) at high temperature, and we can recycle the component of tungsten trioxide in the waste SCR denitration catalyst.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com