Inquiry Europium Tungsten Trioxide Photoelectrode
- Details
- Category: Tungsten Information
- Published on Friday, 22 January 2016 16:11
 Europium is silver and white metallic element, it is used as color TV phosphors, and europium has important applications in laser materials and atomic energy industry. Europium content in the crust is 0.000106%, it is the rarest of rare earth elements. There are two isotopes of europium in the monazite in nature: Europium Eu 151 and 153. Europium oxide is also used in recent years of new X-ray medical diagnostic system stimulated emission phosphor. Europium oxide can also be used in the manufacture of colored lenses and optical filters for magnetic bubble storage devices. It is used in atomic reactor for neutron absorbing materials because its atoms can absorb more neutrons than any other element. In addition, it can be used as color TV phosphors, which emits bright red phosphor to make television screen.
Europium is silver and white metallic element, it is used as color TV phosphors, and europium has important applications in laser materials and atomic energy industry. Europium content in the crust is 0.000106%, it is the rarest of rare earth elements. There are two isotopes of europium in the monazite in nature: Europium Eu 151 and 153. Europium oxide is also used in recent years of new X-ray medical diagnostic system stimulated emission phosphor. Europium oxide can also be used in the manufacture of colored lenses and optical filters for magnetic bubble storage devices. It is used in atomic reactor for neutron absorbing materials because its atoms can absorb more neutrons than any other element. In addition, it can be used as color TV phosphors, which emits bright red phosphor to make television screen.
The preparation method of europium surface-modified tungsten oxide photoelectrode including following steps: 1) preparation of amorphous tungsten oxide thin film: adding H2O2 in Na2WO4 solution to obtain clarification W20n2_ containing electrolyte; and Electrodeposition was carried out to obtain an amorphous tungsten oxide thin film; 2) dropwise europium surface modification method: the surface of the thin film orphous tungsten oxide europium nitrate was added solution of Step I), drying to obtain surface-modified tungsten oxide europium thin film; 3) calcining: the Step 2) surface modification europium obtained tungsten oxide thin film in high-temperature about 450 ° C, cooling and taking out at room temperature to obtain surface-modified tungsten trioxide europium photoelectrode. Its photoelectric performance significantly improved, and the test equipment is simple, it has easy operation, mild conditions, and environmentally friendliness.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Dirty Bombs Shielding Container(1/2)
- Details
- Category: Tungsten Information
- Published on Friday, 22 January 2016 16:10
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Exploration on Tungsten Trioxide Nanorods Growth Principle
- Details
- Category: Tungsten Information
- Published on Friday, 22 January 2016 15:59
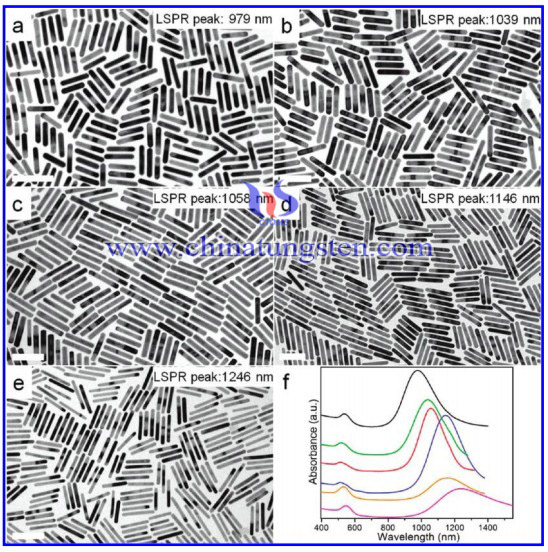 Nanorod is a scale from a few nanometers to hundreds of nanometers rod-like nanoparticles. The surface plasmon resonance wavelength of Nanorods with the aspect ratio can be changed from the visible (550 nm) to near infrared (1550 nm). Nanorod is continuously adjustable, high, and the surface of electric field can high to 10e7 times, which can make a great optical absorption. In scattering cross section, as well as light and heat from 50% to 100%, this performs an adjustable efficiency. Molecular biological properties of nanorods in materials science community being strong concern and caused many materials scientists, biochemists, physicians, physicists, micro electronic engineers and other scientists to the extensive and in-depth research because it is optical, photovoltaic, solar thermal, photochemical.
Nanorod is a scale from a few nanometers to hundreds of nanometers rod-like nanoparticles. The surface plasmon resonance wavelength of Nanorods with the aspect ratio can be changed from the visible (550 nm) to near infrared (1550 nm). Nanorod is continuously adjustable, high, and the surface of electric field can high to 10e7 times, which can make a great optical absorption. In scattering cross section, as well as light and heat from 50% to 100%, this performs an adjustable efficiency. Molecular biological properties of nanorods in materials science community being strong concern and caused many materials scientists, biochemists, physicians, physicists, micro electronic engineers and other scientists to the extensive and in-depth research because it is optical, photovoltaic, solar thermal, photochemical.
In the synthesis process, the selection of the sodium salt as a directing agent can be synthesized nanorods tungsten trioxide, and sulfates as a director are not the product of rod-shaped morphology, which can be inferred sodium ions in the rod formation process, and it plays a decisive role. When the molar ratio of sodium sulfate and sodium tungstate is 4: 1, the product can be seen in XRD diagrams of the various hydrothermal time, when the hot water is heating for longer than 24h, the product is pure hexagonal tungsten trioxide, when the hexagonal tungsten trioxide (200 planes) has emerged, hydrothermal time is 6h, and the (001) plane appears to 12h, which indicates the beginning of the tungsten trioxide hexagonal crystal growth (200) crystal surface is an advantage, (200) have more chance of sodium ion adsorption solution, reducing the chance of tungsten trioxide crystals of (200) plane growth. Making tungsten trioxide another advantage to its crystal face (001) growth, along with the hot water time, the (001) plane growth significantly, tungsten trioxide nanorods can be obtained by hydrothermal after 48h.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Detinning Process of APT Production- Precipitation Method
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 18:10
| APT Supplier: Chinatungsten Online www.ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Beneficiation Technology Progress after Shizhuyuan Method
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 18:08

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Coarse Grain Tungsten Powder Restored from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 17:53
Ultrafine and ultracoarse tungsten carbide (WC) grains are the two development directions of tungsten carbide in recent years. Coarse grain WC powder is the important raw material for coarse grain tungsten carbide. It has been widely used in oil drilling and petroleum perforating element material, anti ballistic missile system and heavy armor penetrator material, because of the less structural defects at high temperature, high hardness, low micro strain and a series of special properties.
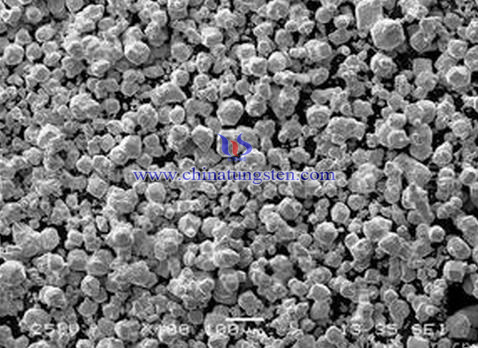
Tungsten powder of coarse particles obtained by one-step reduction method from tungsten trioxide (WO3), in molybdenum wire furnace under the high temperature of 1200℃ at present industrial. However, tungsten particles obtained by this method usually in shape of nearly equiaxed, and the size is relatively small with poor formability, which make it to have to forming by adding organic matter as a binder. And that is not conducive to large products formed and properties controlled. Coarse grain powder was prepared by direct reduction process of ammonium paratungstate (APT) in this paper, and carbonization for preparing WC powder.
The preparation steps are as follows:
1. Use the tungstate solution of 37% concentration; place it in the reaction furnace to heating, evaporation and crystallization to obtain APT crystal;
2. Grinding and crushing the APT, pass through 60 mesh sieve for controlling crystal size;
3. Place APT in a single hydrogen furnace for reduction, set the reducing conditions as follows: hydrogen section flow for 50ml/cm2.min, temperature at 850℃ to 1000℃, keep warm for 90~180min, then tungsten powder obtained;
4. Put tungsten powder into the deionized water, vibration with ultrasonic, cleaned until the solution cleared, drying, grinding, sieving;
5. Mixing tungsten powder and carbon black in a certain proportion and placed in a carbon tube furnace, carbonization under high temperature 1700~1800℃, then milling and sieving to obtain coarse grain WC powder.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Lanthanum Tungsten Electrode
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 17:17
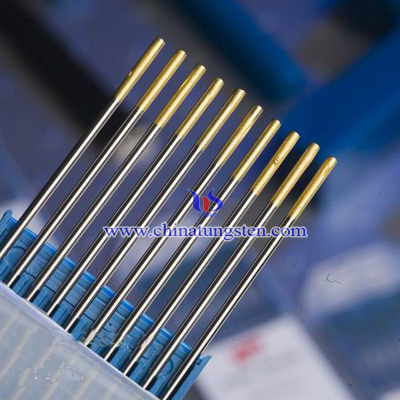
Currently, the tungsten electrode, rare earth tungsten electrode and the composite electrodes are widely used in chemical, electrical, mechanical, construction, aerospace and other fields. But with the development of scientific and technological, tungsten inert gas welding and plasma welding field, there are higher requirements of electrode material and performance have been put forward . The properties of lanthanum tungsten electrodes are superior to thorium tungsten electrode and cerium tungsten electrode which is one of the most widely used electrodes.
Lanthanum tungsten electrodes have good mechanical cutting performance, creep resistance, ductility and high recrystallization temperature is currently the most popular electrode. It was developed from the European country in the 1980’s to replace thorium tungsten electrode. The tip colors of lanthanum tungsten electrodes are varies which depend on the amount of lanthanum oxide (La2O3) doping. When La2O3 doping amount is 0.80 to 1.20%, the tip color is black; when La2O3 doping amount is 1.30 to 1.70%, the tip color is golden yellow; when La2O3 doping amount is 1.80 to 2.20%, the tip color is sky blue.
Lanthanum tungsten electrode is mainly used in DC welding and AC welding. The experiment found that the 1.5% lanthanum oxide content in lanthanum tungsten electrode has similar conductive properties with thorium tungsten electrode which has 2% thorium oxide content. Besides, the burn rate of lanthanum tungsten electrode is smaller and has longer service life. On the other hand, lanthanum tungsten electrode is a non-polluting, non-radioactive green electrode. So it concern in European countries and Japan is very high.
Lanthanum tungsten electrode production method:
1. Add the appropriate amount of La2O3 powder into tungsten trioxide powder, after twice reduction to give W-La2O3 powder. The first reduction temperature is at 600 ~ 700 ℃and time is about 2 to 3 hours; the second reduction temperature is controlled at 800 ~ 970 ℃ and the hydrogen flow is 0.5-2.0m3 / h.
2. After pressing, swaging, stretching, straightening, polishing and other processes obtains the desired electrode rod.
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Cerium Tungsten Electrode
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 17:14

With argon arc welding, plasma welding, cutting, spraying technology continues to update and development, the requirement of electrode materials are increasingly high. In 1972, China first time success developed cerium tungsten electrode, which has a non-radioactive, low-melting, high thermal electron emission ability, arc stability, heat concentration, long life, easy to maintain the shape of the tip and other good properties. What’s more, it can replace thorium tungsten electrodes which has radioactivity.
Cerium tungsten electrode is kind of rare earth tungsten electrodes which added 2% cerium oxide (CeO) into tungsten matrix electrode product production by powder metallurgy, rolling, grinding and polishing and other processes. It is mainly used in low current DC welding, because it has a good arc and welding properties. On the other hand, cerium tungsten usually uses in welded pipes, stainless steel products and small parts welding. Cerium tungsten electrode is not suitable for use in high current conditions, because at high currents cerium oxide will quickly move to the high heat zones, namely the tip of electrode welding, causing cerium oxide uniformity damage. Uniformity of cerium oxide is an important influence factor affecting properties of cerium tungsten electrode, therefore cerium tungsten electrodes is not suitable for high current conditions.
Cerium tungsten electrode material properties are as follows: the work function: 2% Ce-W2.4ev; α-ray dose: 2% Ce-W2.42 × 10-5 curie / kg; oxidation resistance: high oxidation resistance.
Cerium tungsten electrode arc performance: the cathode voltage drop: 2% Ce-W12.0V; cathode spot: the cathode spot and service life are closely linked and it has small cathode spot and loss; electrode emission current density: higher; minimum arc voltage: 12V.
Cerium tungsten electrodes application performance: the burning rate: low; reliability of repeatedly arc and loss rate: arc stability, low loss rate; weld penetration: good.
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Gold-plated Tungsten Cakes
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 17:02

| Tungsten Gold Plated Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CVD Diamond Coated Carbide Pretreatment—Transition Applied (2/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 21 January 2016 17:00
The first part mainly describes the concept and the function of transition layer, this part we introduce some experiments about it. Related researchers certain related technology by using titanium (Ti) and nitrogen (N) ion and the substrate to produce a large number gradually decreased reaction TiCN N ions, so that transition layer containing C, N decrement amount to the surface direction, until the final surface titanium This is to the surface at the time of planting, diamond crystals partially reacted with a titanium Ti titanium carbide TiC, as part of the process of diamond film deposition seed, in order to improve the bonding strength between the diamond film and the buffer layer and the diamond nucleation density; some studies have found that uses TiN and TiCN deposition as the transition layer, it will make diamond film with high purity, but the speed of nucleation is slow. Therefore, they attempt to directly deposited on the surface layer of aluminum carbide substrate, or discontinuous deposit a certain amount of diamond grains, thereby improving the imbalance in thermal expansion coefficient between the TiN / TiC and diamond, making the diamond and speed and adhesion have been significantly improved.
Relevant foreign scholars have used plasma pulsed laser melting method in cemented carbide substrate surface layer of boron nitride (BN) film, which not only eliminates the adverse effects of cobalt Co brought, and significantly improve the binding force between diamond coating layer and tungsten carbide matrix. In addition, Chinese scholars are based on chromium carbide buffer layer deposition CVD diamond coating, and spectral analysis by SEM showed that the deposition process have a more significant impact on the diamond's shape and composition, and ultimately to the complete crystalline, less non-diamond ingredients with carbide substrate binding tightly CVD diamond coating.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com