Inquiry Tungsten Trioxide Infrared Absorption Property
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 18:30
Nano tungsten trioxide is a kind of catalysis semiconductor functional materials; it has stealth features, electrochromic, photochromic gas, light-induced color multifunction, gas, superconductivity, and many other characteristics. Numerous studies show that the surface plasmon resonance can produce semiconductor materials and absorbing specific wavelengths of light. Tungsten oxide nanoparticles increase in certain third phase reduction treatment or cationic, it is capable of accumulating a large amount of free electrons on its surface, so that it has a plasma resonance absorption characteristic of near-infrared light. Since tungsten oxide under anoxic conditions can generate stable Magneli phase and cubic and hexagonal, tungsten structure introducing green copper cations that can form a stable. Therefore, when the reduction treatment can be introduced by a large number of free electrons, tungsten oxide and composite still has a stable structure and physico-chemical properties, and it can remain stable absorption properties in the long sunlight.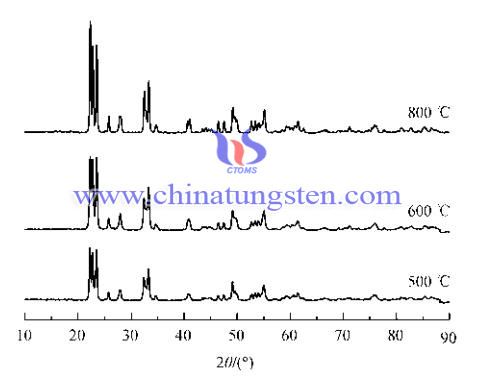
Since tungsten oxide nano powder does not have near-infrared absorption of sunlight, it needs to be subjected to reduction treatment to WO3-x. We take nanometer tungsten trioxide as starting material in a reducing atmosphere, sintering it 1 h at 350--550 ℃ the [volume ratio V (H2): V (N2) = 1: 9], then stop input H2, N2 under protection. The temperature was raised to 800 ℃, and heated at this temperature for 1 h, and then turn off the power so that the temperature dropped to room temperature to obtain WO2.92 and WO2.83. we can find that XRD peak becomes sharp with increasing temperature, the smaller the size of the nanoparticles at lower temperatures, the crystallization is not complete, as the temperature increases, the particle size becomes larger, the corresponding is more complete due to crystallization. The size of the nano-tungsten oxide particles is much smaller than the wavelength of visible light, visible light can maintain transparency.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Detinning Process of APT Production- Precipitation Method
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 18:24
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Heating Selection and Normal Selection of Scheelite
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 18:22
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Detection Arsenic on Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 17:58
 N Carbide and alloy performance is largely determined by the quality of tungsten trioxide. Common methods of preparing tungsten trioxide including the following major steps: first of all, removing Arsenic, silicon, fluorine, arsenic, phosphorus, and molybdenum by solution purification, secondly, the precipitation of artificial scheelite based on calcium chloride solution. Thirdly, using hydrochloric acid to decompose artificial scheelite; finally, the tungsten trioxide is prepared by washed, filtered, dried and calcined in tungstate. This article focuses on the methods for determination of Arsenic in high-purity tungsten trioxide.
N Carbide and alloy performance is largely determined by the quality of tungsten trioxide. Common methods of preparing tungsten trioxide including the following major steps: first of all, removing Arsenic, silicon, fluorine, arsenic, phosphorus, and molybdenum by solution purification, secondly, the precipitation of artificial scheelite based on calcium chloride solution. Thirdly, using hydrochloric acid to decompose artificial scheelite; finally, the tungsten trioxide is prepared by washed, filtered, dried and calcined in tungstate. This article focuses on the methods for determination of Arsenic in high-purity tungsten trioxide.
The definition of Colorimetry is based on the color reaction of the colored compound by measuring or comparing the depth of the colored substance solution. As a method of quantitative analysis, the basic requirements of the Colorimetry are: high sensitivity and selectivity, stable colored compound and different color. The color reaction and control of the reaction conditions is the key for colorimetric analysis.
Determination of Arsenic in High-Purity Tungsten Trioxide Method
Burning the fat high-purity tungsten trioxide at 1400 ℃, and the resultant of Arsenic sulfide reacts with oxygen form Arsenic dioxide. This compound is condensed with formaldehyde, and the purple compound can generate in 560nm colorimetric determination. The experiment of the color, the time and the sample of absorption time can reduce combustion conditions such as blank test. Finally, we can obtain the amount of available mercuric chloride, sodium, chromogenic agent, the influence of magenta and fading. Detection limit of this method is 0.5ppm; the relative standard deviation is ± 4.8%.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Violet Gold-plated Tungsten Bars
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 17:18

| Tungsten Gold Plated Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Platinum-plated Tungsten Commemorative Coins
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 17:16

| Tungsten Gold Plated Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Counterweights For Machine Tools
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 17:11

| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Effect of Tungsten Powder Granularity on Hardness and Conductivity (2/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 16:29
There are also many influencing factors of the electrical conductivity of tungsten copper electrode, such as such as impurities, chemical composition, porosity, and some microscopic structure (including the organizational structure of grain size, W-W connectivity, combined with the strength of grain boundaries, high thermal conductivity copper continuity distribution phase) and so on. Tungsten powder particle size largely affected the tungsten copper alloy electrode porosity and microstructure, thus further affecting its conductivity.
On the one hand, tungsten copper is difficult to be completely densified so that some bores emerge unavoidably, which either alone or connected to each other will have a significant impact on the electrical conductivity of tungsten copper alloy. On the other hand, the finer the grain size of tungsten powder crystal, the situation in the suppression of the process more prone to uneven, making sintered tungsten skeleton channels prone to clogging or closed, resulting in copper Cu-rich region from defects in materials or voids, thereby so that copper was infiltrated feeding inadequate or not effectively reduce the integrity of the network structure after copper infiltration, and ultimately reduce the conductivity. With the increase of the particle size of the tungsten powder, tungsten copper alloy grain size distribution is relatively more uniform, closed pores appear relatively reduced, copper Cu and better connectivity, and the electrical conductivity will gradually rise.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Effect of Tungsten Powder Granularity on Hardness and Conductivity (1/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 20 January 2016 16:28
Different granularity of tungsten powder has different influences on the comprehensive properties of tungsten copper electrode, such as the hardness, the density, the electrical conductivity and micro-structure and so on. Here we highlight the impact of tungsten powder particle size on copper tungsten alloy electrode hardness and conductivity.
Take W-30Cu tungsten copper electrode as an example, the impact of tungsten powder (W) on the hardness and the electrical conductivity of W-30Cu tungsten copper electrode as follow:
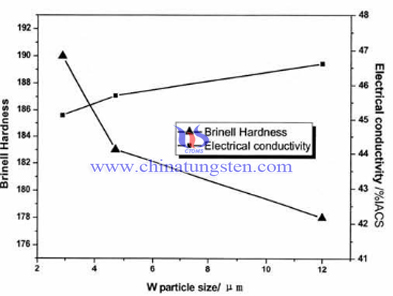
From the figure we change the law, we can see a line of two, one of which is W-30Cu tungsten copper alloy electrode Brinell hardness (HB) changes with tungsten powder particle size curve, and the other is the conductance W-30Cu tungsten copper alloy electrode the rate of change with the tungsten powder particle granularity curve. With the tungsten powder particle size is increased by a 2.9μm to 11-13μm, the conductivity upward trend, while the hardness decreased gradually. Effect of tungsten copper alloy electrode hardness factor not only density, but also the grain granularity.
Due to tungsten (W) is the solid phase in tungsten copper electrode, the finer tungsten granularity, the harder materials. In addition, in the completely compacted conditions, the finer tungsten powder, the smaller size of W grains in alloy and the binding strength of solid phase (W) and binder phase (Cu) with net structure is higher. On the contrary, Tungsten powder particle size is too large, soft phase copper is more susceptible to aggregation, thus tungsten copper alloy electrodes W-30Cu lower hardness. As can be seen from the figure tungsten powder particle granularity is increased from 2.9μm to 11-13μm, hardness decreased from about 190HB to 178HB.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Preparation Blue Tungsten Oxide by Self-Reduction Method from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Tuesday, 19 January 2016 20:17
Because of the shortcomings like cost control and environmental protection in preparation of blue tungsten oxide by traditional reduction method, in order to overcome these problems, domestic and foreign scholars, manufacturers and so on, are trying to develop new methods. Some studies have pointed out that Self-reduction method, use ammonium paratungstate (APT) as raw materials, heating APT in a sealing container to produce blue tungsten oxide. Self-reduction method, just as its name implies, is a method that reactant set under certain conditions in the environment of good airtight, the reaction itself producing some reductive substances which will has a reduction of the oxide to get target product.
Specific steps:
1. Send APT into sealed container, heated to 400℃ to 600℃, APT will decomposition into tungsten trioxide (WO3), ammonia (NH3) and water vapor (H2O);
2. Using tungsten oxide WOX as the good property catalytic for NH3 in a closed container, ammonia is further decomposed into N2 and hydrogen;
3. Control of furnace temperature at 550 to 800℃, set the pressure at 0 to 2 mbar (0~200Pa), restore WO3 to WO2.9 by using hydrogen.
The advantages of using Self-reduction method:
1. Using the ammonia generated from ammonium paratungstate to restore tungsten trioxide, and no need to inlet other reducing gas, thus to reduce the production cost and improve the economic benefit;
2. Eliminate harmful gases such as ammonia and other harmful gas emissions at the same time, reduce air pollution and improve social benefits.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com