Diamond Film and Tungsten Heater
- Details
- Category: Tungsten Information
- Published on Monday, 11 July 2016 16:29
Thermal solution source of tempering wire method - spiral tungsten wire (tungsten heater). However spiral tungsten wire is susceptible to be distorted by the influence of the deposition process. Large area diamond coating requires a relatively uniform temperature field, and deformation of tungsten wire will destroy the uniformity of the temperature field in the original design. Straight tungsten wire as the heater can solve the problem of deformation of spiral tungsten wire sagging. In addition, energy density of the single straight wire is lower than the spiral wire, which can ensure a high pyrolysis energy density. Arrange pyrolysis wire by multiple process can resolve problem of closed-packed of wolfram wire, but due to the large pyrolysis current, so it requires a low-voltage high-current power supply device.
Hardness of diamond film is the highest of solid material, it can reach HV100GPa with a thermal conductivity of 20W • cm-l • K-1, which is 5 times the copper. The resistivity at room temperature is 1016Ω • cm and it can form semiconductor material by doping.
The sample substrate temperature of diamond by tempering wire chemical vapor synthesis method is greatly influenced by the temperature of the pyrolysis wire and the distance of sample substrate. Pyrolysis wire temperature, the distance between pyrolysis wire and the sample substrate and sample substrate temperature are difficult to operate simultaneously at the respective optimum parameters. So operators should use ∅0.3mm diameter tungsten wire, which can reduce sagging of wolfram wire to a minimum degree. Using the independent electric heating device can make the sample maintain a certain temperature, when the sample substrate close to the pyrolysis wire, effective amount of heat of it would radiate to the sample substrate, and it is time to adjust heating power smaller, or it is no need to heating by electric furnace and direct use the flow of cooling water to control the temperature of the sample substrate.
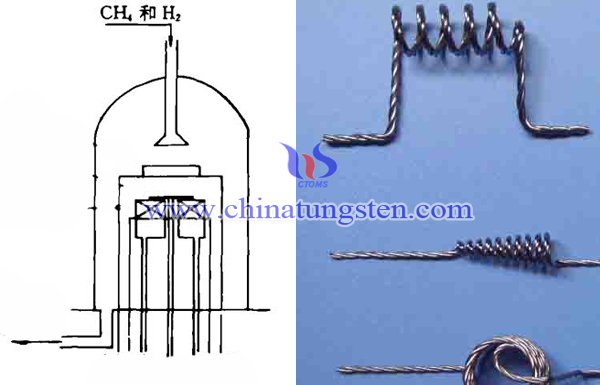
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Coating Machine and Tungsten Heater
- Details
- Category: Tungsten Information
- Published on Monday, 11 July 2016 16:25
The working principle of evaporation coating is that pump gas in coating chamber under 10-2Pa pressure firstly, and then heat the heater to evaporate the material, atomic or molecular of vapor would escape from the surface of the evaporation source and deposit onto the substrate to form a film, which including the basic processes of extraction, evaporation deposition. Coating machine is mainly composed of the coating chamber, a vacuum system, vacuum measurement system and circuit control system. Wherein the tungsten heater is mainly placed in the coating chamber as an evaporator heater.
We can use resistive heating evaporation source - spiral tungsten heater to prepare aluminum film. Spiral tungsten heater is equivalent to a resistor, after electrifying, it would generates heat and its resistivity will increase. When the temperature is about 1000℃, and the resistivity of the evaporation source is five times the room temperature, the evaporation sources of Joule heat is to be able to make aluminum atoms obtain the enough kinetic energy to evaporate.
When the coating chamber pumped to a high vacuum, use the bezel to cover tungsten wire to avoid aluminum would spray onto substrates when pre-melting. Then slowly electrifying and heat tungsten wire, so that aluminum will melt and adhere to the spiral tungsten wire, this process is called pre-melting. The aim is to eliminate impurities which absorb in aluminum, so aluminum would not generate a lot of bleed causing damage to vacuum when evaporation, on the other hand, it also ensures the purity of the film.
Melting temperature of aluminum is 670℃, filiform tungsten wire as evaporation source is because that aluminum has a wetting effect for wolfram wire, evaporation is from the large surface and relatively stable, this wettability is relevant to temperature of tungsten surface. If the temperature is too high, aluminum and tungsten will produce alloys at high temperatures, which can easily cause tungsten have serious distortion even blown.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper CNC Processing — Cutting Fluid Selection and Feeding Route Arrangements
- Details
- Category: Tungsten Information
- Published on Monday, 11 July 2016 15:38
Except that tools and cutting parameters selection, it is also should be noticed that cutting fluid selection and feeding route arrangements in the CNC processing of tungsten copper. Cutting fluid is also called coolant, which is for use in metal cutting, grinding process used to cool and lubricate the cutting tool and the workpiece industrial liquid. Cutting fluid composed by a variety of super-functional additives and has many advantages, such as good cooling performance, lubricating properties, corrosion performance, degreasing cleaning, anti-corrosion features, easy to dilution characteristics. In order to overcome the traditional soap emulsion is easy to smell the summer, hard winter diluted, poor rust problems, and no adverse effects on the lathe paint for ferrous metal cutting and grinding, is currently the most advanced grinding products. Compared to oil saponification, cutting fluid indicators is better, and it has good cooling, cleaning, rust and other characteristics, and with non-toxic, tasteless, non-corrosive to the human body, the device does not corrode the environment without pollution. When cutting a workpiece of tungsten copper will produce high cutting temperature, which is difficult to transfer. So it requires the cutting fluid to enhance the lubricating properties, to reduce cutting forces, greatly improve the durability of the tool, and effectively prevent the sticking knife premature tool wear.
The key process of preventing tungsten copper appearing cracks and dregs in machining is to arrange the feeding route in CNC milling process. Generally, the feeding route follows these principles:
1. Reasonable milling: It can be divided into clockwise and counterclockwise. Milling clock wise is more stable, has shorter cutting distance and it can effectively reduce the bonding chip and improve the surface roughness;
2. To ensure chips out of sample in case of the milling defects by brittle material;
3. To avoid large feeding rate and edge chipping and dregs;
4. When machining plane or a blind slot, select the workpiece surface is not suitable for vertical in feed and possible, choose a slash or helical in feed, to avoid cracking the workpiece;
5. When drilling, it must be close to the other flank of the metal pad, which can effectively control a knife orifice dregs appear.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper CNC Processing — Tool Selection
- Details
- Category: Tungsten Information
- Published on Monday, 11 July 2016 15:36
Tungsten copper is a kind of alloy that composed of tungsten (W) and copper (Cu), which not only has high density, high strength, high melting point, but also has excellent wear and corrosion resistance, has been widely used in aerospace and some high-temperature material fields. But tungsten copper material has bad performance in cutting property and belongs to brittle materials (only at room temperature tensile strength of porous tungsten skeleton 50-120MPa, Cu tensile strength of 240MPa). In the machining process, the tool was badly abrased, and it can easily produce cracks, collapses and other defects. In order to solve these problems, relevant researchers continue to explore in the actual production process of tungsten copper workpiece, especially focused on tool selection, cutting parameters, cutting fluid selection and CNC milling pass route arrangements.
In terms of tool selection, it requires the material has high hardness, good toughness, high strength, excellent hot hardness and heat dissipation. For the best selection of three-flute tungsten carbide end mill, which requires smooth flank, sharp edge, and for multi-point tool should be controlled runout cutting edge. Viewed from the geometry of tungsten copper machining tools, except the requirement of larger posterior horn, the other no special requirements manufactured according to finished tool geometry. For cutting parameters selection, it will produce higher temperature and cutting heat diffusion is not easy, which will accelerate the wear of tool. So choose appropriate cutting speed, cutting depth, feeding rate not only can effectively improve the efficiency, but also can reduce the production costs and extend the service life. In addition, for CNC machining copper tungsten carbide tool cutting workpiece temperature should be controlled between 600 ℃ - 800 ℃. And there is a difference in cutting parameters between tungsten carbide mills and end mills when tungsten copper machining process.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Photocatalyst Photocatalytic CO Synthesizes Formic Acid
- Details
- Category: Tungsten Information
- Published on Friday, 08 July 2016 18:17

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Discoloration Mechanism of WO3 Electrochromic Film
- Details
- Category: Tungsten Information
- Published on Friday, 08 July 2016 18:15
 Electrochromic effect refers to the optical property of materials, which means the color of the materials carry out a stable and reversible changing phenomenon under the effect of the current or the applied electric field. WO3 is the most typical non-organic electrochromic material, the electrochromic film which is made of it has been most studied, with the high coloration efficiency, good reversibility, short response time, long life, low cost, and other advantages, it meets the future development trend of smart materials. This film has a wide range of applications in the fields, like architectural dimming window, rearview mirror and windshields, electrochromic storage device, flat panel displays ect..
Electrochromic effect refers to the optical property of materials, which means the color of the materials carry out a stable and reversible changing phenomenon under the effect of the current or the applied electric field. WO3 is the most typical non-organic electrochromic material, the electrochromic film which is made of it has been most studied, with the high coloration efficiency, good reversibility, short response time, long life, low cost, and other advantages, it meets the future development trend of smart materials. This film has a wide range of applications in the fields, like architectural dimming window, rearview mirror and windshields, electrochromic storage device, flat panel displays ect..| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Working Mechanism of Rare Earth Tungsten Electrode
- Details
- Category: Tungsten Information
- Published on Friday, 08 July 2016 17:40
The working mechanism of rare earth tungsten electrode mainly relies on rare earth tungstate or oxytungstate migration and proliferation in the arcing process, which can achieve the best rare earth optimization ratio in electrode surface. And it can reduce the work function of the electrode surface, promoting electron emission, to improve the welding performance.
Multiple composite rare earth tungsten electrodes at different arcing time have different morphology and rare earth content change. During early arcing, under the action of high-frequency arc, the tip temperature of the electrode is higher, so that the fibrous tissue material shows three different regional characteristics. A area has the highest temperature and the organization presents the semi-molten state. Besides, the surface has tungsten oxide decomposition, so there has small amount of tungsten deposition. What’s more, due to highest temperature of the A area, so the amount of material evaporated within this area is the largest. B area occurred recrystallization and organization is equiaxed. Rare earth in B area presents liquid state and mainly along the grain boundary migrate to the surface, but equiaxed tungsten grains to some certain extent hindered diffusion migration of rare earth, so in this are the diffusion and evaporation is easy to achieve a balance,to form a stable active layer in the electrode surface, which is good for stabilizing arcing and promoting welding performance. C area maintains electrode original processing state of fibrous tissue. In composite electrode, Ce occurred first diffusion to the surface, so during early arcing stage, the role of Ce is the largest. In addition, the diffusion of rare earth in B area and C area is similar and there will take place Ce-based rare earth intergranular diffusion, but since C area tungsten grains exhibit fibrous tissue which parallels with electrode axis, so rare earth mainly migration to the B area.
After stable arcing, the electrode tip temperature is higher than initial arcing stage which has been significantly improved, especially in areas B and C. B area temperature rises to speed the evaporation rate of Ce and reduce the surface content. And the La also diffuse to the surface and it is possible to maintain a larger coverage of the electrode surface, which plays an important role on arcing stable. C area temperature increase can speed La diffusion along the grain boundary rate, so La as the main element along fibrous grain boundary diffuse to the B area, supplementary evaporation of rare earth.
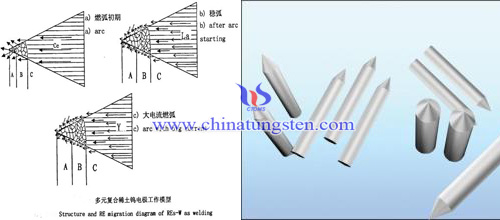
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
The Diffusion of Rare Earth during Tungsten Electrode Arcing
- Details
- Category: Tungsten Information
- Published on Friday, 08 July 2016 17:38
Rare earth can effectively reduce the work function of the electrode surface, lowering the operating temperature of the electrode, which not only can promote electron emission, but also can improve welding performance. Therefore, the largest role of rare earth tungsten electrode is to reduce the surface fuction work to facilitate electron emission, but there are different a lot of different interpretations on electrode emission mechanism. For thorium tungsten electrode, in the arcing process thorium oxide is reduced to elemental thorium, so that the surface of the electrode is covered with a layer of thorium atoms. Besides, the thorium atoms have lower work function, thus the work function of the electrode surface is also lower, which can promote the emission of electrons. But after thermodynamic calculations found that the rare earth tungsten electrode has different emission mechanism with thorium tungsten electrode. In the arcing process, rare earth will not be restored, so that the atomic layer emission mechanism can not explain the diffusion behavior of rare earth in rare earth tungsten electrode.
Through high-temperature simulation to study rare earth diffusion behavior. From the test pattern can be seen at 850 ℃, the content of Ce in the tungsten matrix has far more than its theoretical content. This shows that before heating, Ce has spread to the electrode surface, as the temperature rises, the content slowly increases. At 1100℃ the content can up to the maximum value. However, with temperature increasing, the evaporation rate is greater than the surface diffusion rate, so the content began to decrease.
La began to diffuse at 850 ℃. At 1050 ℃, the surface evaporation rate and the diffusion rate can achieve a balance and the La content reached maximum value 25%. When the temperature rises to 1200 ℃, La evaporation rate is higher than the surface diffusion rate and the content began to decrease.
In the rare earth tungsten electrodes, the mainly content change is La, Ce and O, caused by the change. For O, at 850 ℃ -1200 ℃, O is slowly to increase in electrode surface, reached the highest value at 1000 ℃.
Observed electrode arcing morphology change found the electrode tip has high temperature, which can promote rare earth diffuse to the surface. Ce is the first one began to migrate and the fastest. Therefore, during arcing, Ce first began to spread and formed active layer on the surface to facilitate electron emission, promoting arcing success. The diffusion of La is late and the role of it is stable arcing. The main role of O is in the early arcing. A portion O for forming a surfactant layer, the other can react with the tungsten to form gaseous oxides which may volatilize or deposit to the tip of the electrode, the projection formed, and it is conducive to high-frequency arc and stable arc.
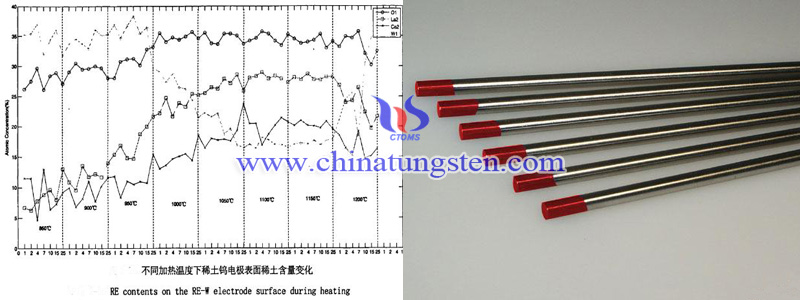
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Powder and Capacitor Dielectric
- Details
- Category: Tungsten Information
- Published on Friday, 08 July 2016 16:30
A method for producing a capacitor includes: a dielectric body (10) composed of basic alumina powder (12) mixed with tungsten powder (14), interposed between a pair of electrodes (16,18) disposed opposite to each other. The method includes the steps of: after a conductive alumina ceramic body has been formed by mixing the tungsten powder (14) with the alumina powder (12), providing the pair of electrodes (16,18) while interposing the conductive alumina ceramic body therebetween, and then intermittently applying an electric current or voltage to the electrodes (16,18), high enough to break conductive paths formed in the conductive body by the tungsten powder (14), so that the conductive body is converted to the dielectric body (10).
The present invention relates to a capacitor, a method for producing the same and method for producing a dielectric body. Particularly, this invention relates to a capacitor wherein a dielectric body formed of a basic dielectric substance mixed with conductive substance is interposed between a pair of electrodes, a method for producing the same and a method for producing a dielectric body obtained by mixing conductive substance in a basic dielectric substance.
A capacitance of a capacitor is given by the following formula: C = (ε0) (ε1) (S/d) wherein
C: capacitance of capacitor
ε0: relative dielectric constant in vacuum
ε1: relative dielectric constant of dielectric body
S: area of dielectric body
d: thickness of dielectric body
To increase the capacitance (C) of the capacitor, it is necessary to enlarge the area (S) of the dielectric body or reducing the thickness (d) of the dielectric body.
In Japanese Unexamined Patent Publication (Kokai) No. 3-87091, a method for producing an alumina ceramic circuit board is proposed wherein a green sheet formed of aluminum powder as a ceramic material added with molybdenum powder and/or tungsten powder in a range between 5 and 50% by weight is fired at a predetermined temperature.
According to the method disclosed in this Patent Publication, it is possible to easily form a capacitor within the alumina ceramic circuit board.
In this production method, if an amount of molybdenum powder and/or tungsten powder gradually increases, the relative dielectric constant of the resultant ceramic dielectric body also increases.
However, if the added amount of conductive substance exceeds 50% by weight, a resistance of the obtained ceramic body suddenly decreases to exhibit electrical conductivity.
Accordingly, in the method disclosed in the Patent Publication, it is possible to increase the mixed amount of conductive substance to about 50% by weight, whereby the relative dielectric constant of the resultant dielectric body is at most 17 or so.
On the other hand, there has been a great demand for a dielectric body having a higher dielectric constant in a circuit board of alumina ceramic or the like. If a dielectric body having a higher dielectric constant could be formed, effective means would be obtained for forming a capacitor having a high capacitance in the circuit board.
Inventors of the present invention studied to achieve the above object. As a result, a conductive ceramic body was molded by firing at a predetermined temperature a green sheet formed of alumina powder mixed with tungsten powder of 60% by weight. Then, a pair of electrodes were provided opposite to each other while interposing the molded ceramic body. It was found that the ceramic body is converted to a dielectric body by applying a high current or voltage in a pulsed manner to the electrodes. Thus, the present invention was completed.
US-A-5144529 discloses a capacitor formed by dispersing needle-like metal particles coated with an oxide layer in a synthetic resin film. The particles are oriented parallel to electrodes on the resin film by a magnetic field, prior to curing of the resin film.
Accordingly, in a first aspect, the invention consists in a capacitor including a dielectric body interposed between a pair of electrodes disposed opposite to each other, said dielectric body being formed by firing or curing a conductive body formed from a mixture of a basic dielectric substance and a conductive substance,
characterized in that conductive paths provided by the conductive substance in the conductive body are electrically broken by intermittently applying an electric current or voltage to the electrodes.
In a further aspect, the invention consists in a method for producing a capacitor including a dielectric body composed of a basic dielectric substance mixed with conductive substance, interposed between a pair of electrodes disposed opposite to each other, said method comprising the steps of: Forming a conductive body by mixing the conductive substance with the dielectric substance,providing the pair of electrodes on opposite sides of the conductive body, firing or curing the conductive body, and intermittently applying an electric current or voltage to the electrodes whereby conductive paths formed in the conductive body by the conductive substance are electrically broken, so that the conductive body is converted to the dielectric body.
In a further aspect, the invention consists in a method for producing a dielectric body wherein a conductive substance is mixed with a basic dielectric substance comprising the steps of: forming a conductive body by mixing the conductive substance with the dielectric substance, interposing the conductive body between a pair of electrodes, firing or curing the conductive body, and intermittently applying an electric current or voltage to the electrodes to electrically break conductive paths formed in the conductive body by the conductive substance, so that the conductive body is converted to the dielectric body.
According to the present invention having such structures, it is possible to obtain a ceramic or plastic body exhibiting the conductivity by mixing a conductive substance exceeding 50% by weight or 15.9% by volume in a ceramic material exhibiting the dielectric property.
It is also possible to form a conductive body capable of being converted to a dielectric body in a circuit board of alumina ceramic at the same time as the circuit board of alumina ceramic is fired, by using, as ceramic material, alumina powder and, as conductive substance, tungsten powder, tungsten oxide powder, molybdenum powder or molybdenum oxide powder.
According to the present invention, the conductive body is formed by mixing a large amount of conductive substance in the dielectric substance. Conductive paths are formed within this conductive body.
Then, the conductive body is converted to the dielectric body by intermittently applying a high current or a high voltage to the electrodes while interposing the conductive substance between them to electrically break the conductive paths.
The conductive body converted to the dielectric body as mentioned above has a higher relative dielectric constant compared with that of a dielectric body formed by mixing therewith conductive substance of an amount capable of substantially maintaining the insulating property of the dielectric body.
It is surmised that the reason therefor is that an extremely thin dielectric layer, compared with a dielectric layer in a dielectric body wherein a smaller amount of conductive substance is mixed with the dielectric substance, is formed on the surface of a particle of the conductive substance forming a conductive path.
Thus, according to the present invention, it is possible to obtain the dielectric body having a higher relative dielectric constant even though dielectric substance having a lower relative dielectric constant is used.
A dielectric body forming a capacitor according to the present invention can be obtained by mixing conductive substance in dielectric substance.
Ceramic materials or organic insulating materials such as plastic or others may be used as the dielectric substance. Particularly, alumina powder, aluminum nitride powder or glass powder can be suitably used as the ceramic material.
The conductive substance suitably mixed with such dielectric substances is preferably selected while taking the relationship with the dielectric substance into account. For example, when a dielectric substance capable of forming a ceramic body through a high temperature firing is used, a ceramic material such as alumina powder, or a powder of metal having a melting point in the vicinity of that of the ceramic material is favorably used, such as tungsten powder, tungsten oxide powder, molybdenum powder or molybdenum oxide powder.
On the other hand, when an organic insulating body such as plastic or glass powder is used as the dielectric substance, powder of Au, Cu or Ag is preferably used, which has a lower melting point than that of tungsten powder or the like but is excellent in the electrical conductivity.

| Tungsten Powder Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Comparison of Tungsten (Tungsten Heater) and Molybdenum for Heater Material
- Details
- Category: Tungsten Information
- Published on Friday, 08 July 2016 16:14
 Common metals that have the lowest melting point include tin (232 ℃), lead (327 ℃), magnesium (651 ℃), zinc (419 ℃), antimony (630 ℃), aluminum (660 ℃), copper (1083 ℃), etc. However, under the low temperature (below 0℃) tin will destruct itself, and lead is soft with the low intensity, no metallic magnesium and zinc has active chemical property, which are easy to react with the non-metallic. Antimony is brittle and easy to volatilize when heated. In short, those materials are not suitable for coating of aluminum. Aluminum and copper have the metallic luster with high stability and low cost, so aluminum and copper are the most ideal coating materials. Tungsten and molybdenum are easy to oxidized at high temperatures, which is the key issue for them as a promising high-temperature structural materials.
Common metals that have the lowest melting point include tin (232 ℃), lead (327 ℃), magnesium (651 ℃), zinc (419 ℃), antimony (630 ℃), aluminum (660 ℃), copper (1083 ℃), etc. However, under the low temperature (below 0℃) tin will destruct itself, and lead is soft with the low intensity, no metallic magnesium and zinc has active chemical property, which are easy to react with the non-metallic. Antimony is brittle and easy to volatilize when heated. In short, those materials are not suitable for coating of aluminum. Aluminum and copper have the metallic luster with high stability and low cost, so aluminum and copper are the most ideal coating materials. Tungsten and molybdenum are easy to oxidized at high temperatures, which is the key issue for them as a promising high-temperature structural materials.
Under a temperature below 300℃, molybdenum is relatively stable. A blue oxide film would formed over the surface if the temperature is higher than 300℃. The oxide film will evaporate if the temperature is higher than 500℃, It can be concluded that the higher the temperature, the faster the evaporation. White smoke MoO3 would be formed when over 700℃.
Wolfram of tungsten heater begins to oxidize in air at 400 ~ 500℃. If the temperature is above 600℃, WO3 thin film would be formed on the surface, because the specific volume of WO3 is greater than the base, tungsten wire would crack after reaching this temperature, increase the speed of oxidation. If the temperature is over 900℃, WO3 would volatilize, then metal would be oxidized at a faster rate.
Therefore, the choice of tungsten or molybdenum as a heater material should be determined in according to the plated material, and to appropriately control the temperature.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com