Tungsten Copper / Bronze Self-moving Arc Contact
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 15:45
Tungsten copper contact materials have excellent arc erosion resistance, welding resistance and high strength, so it has been widely used in various circuit breakers, vacuum load switch and transformer conversion switch, especially tungsten copper / copper alloy arc contact. With the increasing high-voltage switch level and the switchgear miniaturization trend, the requirements of self-moving arc contact has increased. And with 550KV high-voltage power grid running, the properties requirements of tungsten copper / bronze (QCr0.5) self-moving arc contact has become more and more strict. After processing, self-moving arc contact conducted line cutting or milling that inner diameter will has a significant modification due to the pre-machining process stress and has outer or inner expansion according to different nature of stress. This deformation will affect the movable arc contact with the stationary arcing contact elasticity, lower voltage electrical arcing contacts in the breaking process reliability, thus affecting the overall performance of high-voltage electrical. In addition, Mechanical correction method is only effective on short-term, and it will be installed after the re-deformation with stress relaxation, thereby reducing the overall contact reliability.
Its basic process is: First of all, adopt wet mechanical to mix Cu powder and tungsten powder, and put the mixed powder granulated in the suppression of press-molding mold cavity; the compacted into a green body is sintered graphite boat infiltration in an atmosphere sintering furnace, copper-tungsten alloy prepared by the contact portion, followed by partial sintering conductive rod connected to the contact portion of the copper-tungsten alloy and chrome bronze material to obtain copper tungsten alloy / chrome bronze overall movable arc contacts the workpiece; afterwards, solid solution treatment to tungsten copper / bronze self-moving arc contacts and then roughing, followed by aging treatment; finally, finishing and long the circumference of the workpiece after the partial aliquot line cutting, to obtain a copper tungsten / chromium bronze overall self-elastic movable arc contact.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Copper Guide Plate
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 15:22
Tungsten copper composite material has high hardness, high strength, high melting point and excellent wear and corrosion resistance, which has been widely used in many high temperature and corrosive material fields. As the key part of rolling mill, tungsten copper guide plate plays an important role in passing in and out of the groove, which performance and service life has an great effect on the efficiency directly. When the guide plate works, contact with hot rolling and doing a relatively high speed movement, the main failure modes include high temperature oxidation wear and fatigue spalling. So it requires the materials for guide plate not only has good high-temperature oxidation resistance, high temperature wear resistance, but also has excellent toughness and impact resistance, which all suits the characteristics of tungsten copper composite materials.
Compared with alloy steel guide plate, tungsten copper plate guide and guard by casting penetration surface alloying alloy powder previously fixed at a specific location and casting so that the surface has a special microstructure and properties of wear-resistant, heat-resistant high-alloy layer and the substrate itself has a good toughness to withstand impact. Adopt new non-binder alloy powder infiltration process, to overcome the current general casting penetration surface alloying process stability is poor, poor surface quality, combined with surface layer or infiltration casting porosity and other shortcomings easily appears; using a special alloy powder embedded processing and packaging reasonable alloy powder ratio, thus ensuring the casting penetration surface alloyed layer and the substrate is a good combination; substrate itself has sufficient strength and toughness, combined with the antioxidant properties of the alloy layer and anti-wear properties of high temperature. Overall, surface alloying process has many advantages, such as simple equipment, short production cycle, low production cost, the parts not easily deformed and good thickness of the treated layer.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide Nanoparticle as a Catalyst for Malonic Acid Ester Synthesis via Ozonolysis
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 13:36
 Ozonolysis is of great interest to synthetic organic chemistry because it is one of the most efficient tools for oxidatively cleaving carbon-carbon double bonds. Ozonolysis is generally used to prepare biologically active molecules. Therefore, the reactions between ozone and organic compounds continue to be a subject of significant interest from mechanistic, synthetic, and environmental perspectives. The importance of O3 reactions with alkenes in the troposphere and solution has led to many experimental and theoretical studies of their kinetics and mechanism.
Ozonolysis is of great interest to synthetic organic chemistry because it is one of the most efficient tools for oxidatively cleaving carbon-carbon double bonds. Ozonolysis is generally used to prepare biologically active molecules. Therefore, the reactions between ozone and organic compounds continue to be a subject of significant interest from mechanistic, synthetic, and environmental perspectives. The importance of O3 reactions with alkenes in the troposphere and solution has led to many experimental and theoretical studies of their kinetics and mechanism.
Scientists addresses tungsten oxide nanoparticle syntheses for use as a catalyst in the novel one-step synthesis of malonic acid ester. Malonic acid ester is directly synthesized and esterified via the ozonolysis of palm olein (palm oil fraction), which comprises 10% linoleic acid as an unsaturated fatty acid. The main advantages of using tungsten oxide nanoparticles as the catalyst for this esterification are to shorten the reaction time. Using tungsten oxide nanoparticles also has minor advantages such as simple synthetic operation, excellent yields, and recyclability. The structure and size of the tungsten oxide nanoparticles were investigated via field emission scanning electron microscopy (FE-SEM) and X-ray powder diffraction (XRD). The malonic acid ester was spectroscopically characterized via gas chromatography mass spectroscopy (GC-MS).
The study used a new approach to synthesize malonic acid ester via the direct ozonolysis of palm olein, where the fine bubbles of ozone played an active role to cleave the double bonds. This method was used for the first time with a tungsten trioxide catalyst for the esterification of malonic acid and had the advantages of synthetic simplicity, an excellent 10% yield, short 2 h reaction time, and recyclability. Malonic acid ester was characterized using mass spectroscopy. The prepared nanoparticles have a spherical shape and diameter of 24 nm have been investigated by the FESEM & XRD, respectively.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Porous Orthorhombic Tungsten Oxide Thin Films Applied in Electrochromic devices and Photochromic Devices
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 13:26
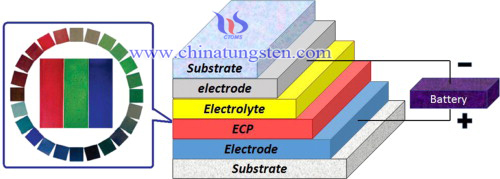 Tungsten oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten. It is obtained as an intermediate in the recovery of tungsten from its minerals. The tungsten oxide nanoparticles can be applied in many areas. They can be used in colorant and analysis reagent of chinaware, used in producing metal tungsten material, gas sensors, fire-proofing fabrics, imaging; large-area displays, catalysts; ceramic pigments; humidity sensors, infrared switching devices; high-density memory devices and so on.
Tungsten oxide, also known as tungsten trioxide or tungstic anhydride, WO3, is a chemical compound containing oxygen and the transition metal tungsten. It is obtained as an intermediate in the recovery of tungsten from its minerals. The tungsten oxide nanoparticles can be applied in many areas. They can be used in colorant and analysis reagent of chinaware, used in producing metal tungsten material, gas sensors, fire-proofing fabrics, imaging; large-area displays, catalysts; ceramic pigments; humidity sensors, infrared switching devices; high-density memory devices and so on.
orthorhombic tungsten oxide (o-WO3) thin films, stabilized by nanocrystalline anatase TiO2, are obtained by a sol–gel based two stage dip coating method and subsequentannealing at 600℃. An Organically Modified Silicate (ORMOSIL) based templating strategy is adopted to achieve porosity. An asymmetric electrochromic device is constructed based on this porous o-WO3 layer. The intercalation/deintercalation of lithium ions into/from the o-WO3 layer of the device as a function of applied coloration/bleaching voltages have been studied. XRD measurements show systematic changes in the lattice parameters associated with structural phase transitions from o-WO3 to tetragonal LixWO3 (t-LixWO3) and a tendency to form cubic LixWO3 (c-LixWO3). These phase transitions, induced by the Li ions, are reversible, and the specific phase obtained depends on the quantity of intercalated/deintercalated Li. Raman spectroscopy data show the formation of t-LixWO3 for an applied potential of 1.0 V and the tendency of the system to transform to c-LixWO3 for higher coloration potentials. Optical measurements show excellent contrasts between colored and bleached states. An alternate photochromic device was constructed by sensitizing the o-WO3 layer with a ruthenium baseddye. The nanocrystalline anatase TiO2 in the o-WO3 layer has led to an enhanced photochromic optical transmittance contrast of ∼51% in the near IR region. The combination of the photochromic and electrochromic properties of the synthesised o-WO3 layer stabilized by nanocrystalline anatase TiO2 opens up new vista for its application in energy saving smart windows.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Powder Preparation Using Violet Tungsten oxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 13:14
Tungsten carbide is a chemical compound containing equal parts of tungsten and carbon atoms. In the tungsten carbide powder, carbon atoms will embed in the interstice of tungsten metal lattice, which will not destroy the lattice of original metal and will form interstitial solid solution. Therefore, it can be also called interstitial compound or insertion compound, which is mainly used in producing cemented carbide due to its high hardness. As tungsten carbide powder has large application range, it has given rise to the waves of researching the preparation of tungsten carbide powder. Violet tungsten oxide can be used in producing tungsten carbide powder, this is because it has single-crystal structure, abundant crack as well as its interior is composed by loose needle-like or rod like particles, which will be beneficial for making tungsten carbide powder that has superior quality. In the preparation of tungsten carbide powder by violet tungsten oxide, violet tungsten oxide is used as the raw material, which will be reduced by hydrogen in the four tube furnace according to the traditional technology, in which the raw material load will be 600g, the pushing speed will be 20min/load and the hydrogen flow will be 30.0m3/h. Under fully unified technology, the reduction process of various oxides will be carried out in the same furnace.
The tungsten powders that have been generated in the furnace will be respectively fit with carbon under same carbon content, which will be mixed evenly and will be carbonized in the same carbide furnace under the carbonization temperature of 1100 degrees Celsius to 1400 degrees Celsius, thus the tungsten carbide powder can be obtained. Tungsten carbide powder that is produced by violet tungsten oxide will have small and even particle size as well as narrow particle distribution, besides, it has no structural agglomerate, which can be made into superfine cemented carbide that has high strength and high hardness.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide in Polymer Electrolyte Fuel cell Electrodes
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 11:57
There is an experiment the scientists have done. Thin films of Tungsten oxide and Pt on Tungsten oxide were evaporated onto the microporous layer of a gas diffusion layer (GDL) and served as model electrodes in the polymer electrolyte fuel cell (PEFC) as well as in liquid electrolyte measurements. In order to study the effects of introducing Tungsten oxide in PEFC electrodes, precise amounts of Tungsten oxide (films ranging from 0 to 40 nm) with or without a top layer of Pt (3nm) were prepared.
The structure of the thin-film model electrodes was characterized by scanning electron microscopy and X-ray photoelectron spectroscopy prior to the electrochemical investigations. The impact of Nafion in the electrode structure was examined by comparing samples with and without Nafion solution sprayed onto the electrode. Fuel cell measurements showed an increased amount of hydrogen tungsten bronzes formed for increasing Tungsten oxide thicknesses and that Pt affected the intercalation/deintercalation process, but not the total amount of bronzes. The oxidation of pre-adsorbed CO was shifted to lower potentials for Tungsten oxide containing electrodes, suggesting that Pt-Tungsten oxide is a more CO-tolerant catalyst than Pt. For the HOR, Pt on thicker films of Tungsten oxide showed an increased limiting current, most likely originating from the increased electrochemically active surface area due to proton conductivity and hydrogen permeability in the Tungsten oxide film.
From measurements in liquid electrolyte it was seen that the system behaved very differently compared to the fuel cell measurements. This exemplifies the large differences between the liquid electrolyte and fuel cell systems. The thin-film model electrodes are shown to be a very useful tool to study the effects of introducing new materials in the PEFC catalysts. The fact that a variety of different measurements can be performed with the same electrode structure is a particular strength.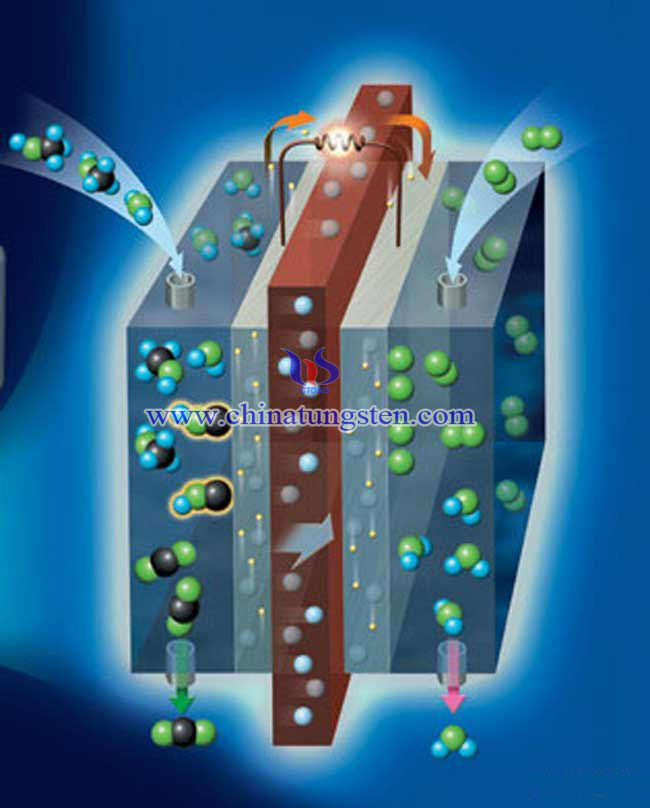
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten/Tungsten Oxide pH Sensing Electrode for High Temperature Aqueous Environments
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 11:51
The tungsten/tungsten oxide electrode was investigated as a pH sensor over a wide range of pH at temperatures from 200 to 300 ℃ and was compared with the yttria-stabilized zirconia membrane electrode. The tungsten/tungsten oxide electrode shows a Nernstian pH response within the pH range of 2 to 11 and exhibits stable and reproducible potentials. In solutions with pH values lower than 2 were observed deviations from Nernstian behavior. The potential of the electrode is not significantly affected by the redox properties of the system, as established by the presence of oxygen or hydrogen in the
The reliable determination of pH in high temperature aqueous environments is of primary importance for the development of corrosion prevention technologies in the thermal power industry. A knowledge of pH is also important for developing an understanding of various aspects of high temperature water chemistry and geochemistry.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Glass
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 11:36
 There is a new nano technology innovation that greatly improves the strength, impact resistance and conductivity of Glass. We call it "Tungsten Glass"
There is a new nano technology innovation that greatly improves the strength, impact resistance and conductivity of Glass. We call it "Tungsten Glass"
By combining the attributes of (borosilicate-based glass) or pyrex glass with tungsten and carbon multi layered nanotubes during the kiln melting process, Tungsten Glass is created. This advanced super glass is perfect for the demanding role associated with smart phones and tablets. Manufactures of these globally distributed products are keenly aware of the problems associated with the fragile glass they used on the front of their products.
The displays of electronic devices such as smart phones and tablet computers are commonly protected via glass that is specifically designed to resist scratches as well as reasonable amounts of damage from drops. However, the protection provided by protective glass is rather minimal and the glass is subject to significant cracking and other damage that can hinder convenient usage of an electronic device. In addition to compromising one’s ability to use an electronic device, replacement of damaged protective glass is often expensive. Tungsten Glass seeks to improve upon conventional protective glass for use in protecting electronic displays and similar applications. The present invention is a borosilicate-based glass that is infused with tungsten and carbon nanotubes. The tungsten glass innovation enhances the protective qualities of the glass by providing improved resistance to impact and scratching.
Multi layered nanotubes have great spring type resistance and can absorb impact and return to their original shape. This spring like feature reduces various harmonic vibration in mechanical movements, due to it’s ability to absorb impact and rebound.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Oxide and Heteropoly Acid-Based Systems for the Catalysts in PEM Fuel Cell Cathodes
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 11:29
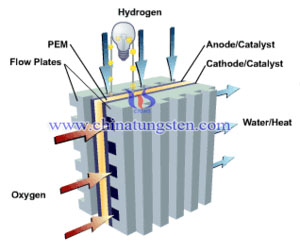 There is an experiment done by the scientists. The objective is to improve electrocatalyst and membrane electrode assembly (MEA) durability and activity through the use of Pt/tungsten oxide and Pt/HPA-C (heteropoly acid) modifications to approach automotive proton exchange membrane (PEM) fuel cell activity targets (0.44 mA/mg Pt) and durability targets (5,000 h/10 y).
There is an experiment done by the scientists. The objective is to improve electrocatalyst and membrane electrode assembly (MEA) durability and activity through the use of Pt/tungsten oxide and Pt/HPA-C (heteropoly acid) modifications to approach automotive proton exchange membrane (PEM) fuel cell activity targets (0.44 mA/mg Pt) and durability targets (5,000 h/10 y).
Our target is to synthesize alternative supports such as tungsten oxide and HPA-functionalized carbon blacks and to evaluate them for improved corrosion resistance while maintaining or improving activity in comparison to conventional Pt/C supports. Studies are first being conducted in rotating disk electrode (RDE) setups due to the small quantity of materials synthesized and will be followed by testing in fuel cells.
The mass activity of ALD-deposited Pt/ tungsten oxide improved to ~175 mA/mg when measured in RDE halfcells. This activity is a significant improvement but falls short of the activity of baseline Pt/C. Pt/ tungsten oxide was scaled up to g quantities using ALD deposition on HWD tungsten oxide; however, the scaled-up Pt/ tungsten oxide -C did not show these improvements. The lower activity of Pt/ tungsten oxide is primarily due to the low electronic conductivity of the tungsten oxide as seen by the doubling in activity with the addition of 50 wt% carbon black. Based on these results, further work on Pt/ tungsten oxide has been given a No-Go and alternative paths are being pursued.
Pt/ tungsten oxide was scaled up to gram quantities using the ALD technique and evaluated for activity in RDE setups.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
The Reason of the Tungsten Heater Large Loss in the Aluminizing Process ——Breaking
- Details
- Category: Tungsten Information
- Published on Wednesday, 13 July 2016 11:22
 If the tungsten heater loss is large, it would cause a lot of waste of material. Because that the breaking or aging of heater element would cause aluminum evapotranspiration incomplete, thus affecting the quality of the final product. What’s more due to the frequent breaking of the heater element, the operators have to install and change the heater element constantly, which would increase the workload of the operators and reduce utilization of the equipment, and ultimately affect the entire production.
If the tungsten heater loss is large, it would cause a lot of waste of material. Because that the breaking or aging of heater element would cause aluminum evapotranspiration incomplete, thus affecting the quality of the final product. What’s more due to the frequent breaking of the heater element, the operators have to install and change the heater element constantly, which would increase the workload of the operators and reduce utilization of the equipment, and ultimately affect the entire production.
The reasons for wolfram heater loss generally include breaking, aging and oxidation of the heater element. While heater element is hard, it is also very crisp to break, operators should be careful of it when transport from storage to avoid the impact of foreign objects. The improper or uneven stress of assembly process would lead to break, which requires the operator to be very careful. When assembling the heater element, the force should be uniform, operators can use a torque wrench to install the reagent, and make sure that both ends of the clamp are parallel state. The wolfram heater should be handled gently, it should not be hit with a hard object, so that it would not get any damage. The best way is to solve this problem is to improve the assembly proficiency of operators.
In addition, when heating the heater element, it would have heat expansion, if the heater element is nipped too tight, there is no room for retractable, then high temperature would lead to the increase of length of the heater element, finally, it would bend and has deformation. When the bending last for a long time, the heater element would break, such breakage is generally called as intermediate fracture. However, if the fixture does not tighten heater, the effective power would loss too much, thus affecting the evaporation effect. Operators can add spring on the fixture to solve this problem.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |




 sales@chinatungsten.com
sales@chinatungsten.com