WO3 Enhances SCR Denitration Catalyst Activity and Thermal Stability
- Details
- Category: Tungsten Information
- Published on Wednesday, 23 March 2016 19:53
- Written by chunyan
- Hits: 251
Tungsten trioxide can be used in lots of fields, the mainly use is calcined to produce tungsten and tungsten carbide powder, then to produce carbide products, such as cemented carbide cutting tools; at the same time, the tungsten trioxide is also very good shielding material, and using in X-ray shielding and fireproof fabric frequently; in addition, another important use of tungsten trioxide is used as raw material for producing SCR denitration catalyst.

Catalyst is the core part of the SCR technology; its effective operation determines SCR denitration system efficiency and economy. The mainly active substances of SCR method denitration catalyst are titanium dioxide, vanadium pentoxide, tungsten trioxide, molybdenum trioxide, etc., among them titanium dioxide is the effective carrier. Vanadium oxide can disperse uniformly on the titanium dioxide surface, and anatase titanium dioxide is the highest activity in industry. Vanadium pentoxide being the most important active ingredient in SCR denitration catalyst, the amount of it is different in different catalysts, but the higher vanadium pentoxide content leads to a higher activity of the catalyst.
However, due to the extremely high activity of vanadium pentoxide, it will oxidize sulfur dioxide into sulfur trioxide, which will affect SCR denitration catalyst efficiency. Therefore, a high level of vanadium pentoxide is generally not recommended, the many using of denitration catalyst contents 2%~5% of vanadium pentoxide. The introduction of tungsten trioxide plays a major role in enhancing the activity and increasing the thermal stability of the denitration catalyst. That is because tungsten trioxide can limit catalyst being sulfated, the SCR denitration catalyst commonly used containing 5%~10% of tungsten oxide. Molybdenum trioxide takes the main role of improving catalyst activity and preventing catalyst being poisoned by As in the flue gas. But because molybdenum trioxide disadvantages to catalyst forming, and reducing the ratio of honeycomb catalyst end product, so molybdenum trioxide is mainly used in producing flat-catalyst.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Waste Cellular SCR Denitration Catalyst Recovers WO3
- Details
- Category: Tungsten Information
- Published on Wednesday, 23 March 2016 19:51
- Written by chunyan
- Hits: 264
Cellular SCR denitration catalyst will partly or entirely loss activity after being used for some time, thus cannot to be effective and bring out a lot of waste catalysts. If we don’t recover the noble metals such as titanium, tungsten, and vanadium in the catalysts, it will inevitably result in resources wasted, environmental pollution and costs increased.
If we can change waste into treasure, which means recovering the waste catalysts as raw materials for producing catalyst, making these materials circulating, we guess it would be a very good approach. A method that recovering powder containing tungsten trioxide from waste cellular SCR denitration catalyst have been pointed out. The steps are as follows:
1. Crushing: Crushing the waste SCR denitration catalyst into 30~50mm pieces by a crusher, and also clearing away the ash by a shaker;
2. Cleaning: Cleaning the catalysts after crushing by an ultrasonic for 1 hour, and then washing by water;
3. Drying: Drying the catalyst after cleaning in an oven at 100-150°C for 10~15 hours;
4. Grinding: Grinding the dried catalyst into powder with particle size of 5-50um, then the waste SCR denitration catalyst containing tungsten trioxide obtained.
The recovered waste SCR denitration catalyst containing tungsten trioxide can be reused as the material for producing fresh catalyst, thus to achieve the goal of recycling. The implementation is: mixing the raw materials titanium dioxide, glass fibers, waste SCR denitration catalyst recycled material, tungsten trioxide, vanadium pentoxide and deionized water, after kneading, extruding, drying and calcining to prepare SCR denitration catalyst.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CsxWO3 Nanorods Water Controlled-Release Process
- Details
- Category: Tungsten Information
- Published on Wednesday, 23 March 2016 18:32
- Written by xinyi
- Hits: 270
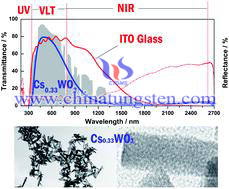
A systematic investigation of the synthesis of homogenous CsxWO3 nanorods by a designed water-controlled release process was carried out. The results revealed that the uniform rod-like CsxWO3 nanoparticles with a Cs/W atomic ratio of ca. 0.33 can be obtained by using 20 vol% CH3COOH–80 vol% CH3CH2OH mixed solution as a reaction solvent at 240 °C for 20 h.
The morphology of products were changed depending on the speed of water-releasing process, meanwhile, the Cs/W atomic ratio could be controlled by both the amount of released water and the reaction temperature. CsxWO3 nanorods showed a high transmittance in the visible light region and excellent shielding ability of near infrared (NIR) lights, indicating that CsxWO3 nanorods have a suitable characteristic as solar filter applications.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Homogeneous CsxWO3 Nanorod on Cancer Therapy
- Details
- Category: Tungsten Information
- Published on Wednesday, 23 March 2016 18:43
- Written by xinyi
- Hits: 268
Recently, photothermal ablation therapy (PTA) employing near-infrared radiation (NIR) has been extensively investigated as an emerging modality for cancer management. However, the clinical translation of this promising approach is limited by the lack of PTA agents with broad NIR absorption, low cost and high photothermal conversion efficiency. Herein, the developed PEGylated homogeneous CsxWO3 nanorods (a mean size ∼69.3 nm × 12.8 nm) with broad photo-absorption (780-2500 nm) as a novel NIR absorbent for PTA treatment of human cancer. The prepared CsxWO3 nanocrystals displayed strong near-infrared optical absorption with a high molar extinction coefficient (e.g. 4.8 × 10(10) M(-1) cm(-1) at 980 nm), thus generated significant amounts of heat upon excitation with near-infrared light. The PTA study in two human carcinoma cell lines (i.e. A549 lung cancer cells and HeLa ovarian cancer cells) demonstrated that CsxWO3 nanorods can efficiently cause cell death via hyperthermia induced lysosome destruction, cytoskeleton protein degradation, DNA damage and thereafter cellular necrosis or apoptosis.
The study also confirmed the migration of healthy cells migrated from unirradiated areas to dead cell cycle, which is essential for tissue reconstruction and wound healing after photodestruction of tumor tissue. The prompted results reported in the current study imply the promising potential of CsxWO3 nanorods for application in PTA cancer therapy.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CsxWO3: N2 Annealing Effects on Heat Shielding Properties
- Details
- Category: Tungsten Information
- Published on Wednesday, 23 March 2016 18:25
- Written by xinyi
- Hits: 270
Cesium tungsten bronze (CsxWO3) powders were synthesized by hydrothermal reaction at 190 °C by using sodium tungstate and cesium carbonate as raw materials, and the effects of N2 annealing on the microstructure and near-infrared (NIR) shielding as well as heat insulation properties of CsxWO3 were investigated. The results indicated that the synthesized CsxWO3 powders exhibited hexagonal Cs0.32WO3 crystal structure, and subsequent N2 annealing could further improve the crystallinity of CsxWO3 particles.
Moreover, the NIR shielding and heat insulation properties of CsxWO3 could be further improved after N2 annealing at appropriate temperature for a period of time. Particularly, the 500 °C-annealed CsxWO3 products in the N2 atmosphere showed the best NIR shielding and heat insulation properties. When the N2 annealing temperature was higher than 700 °C, the NIR shielding properties decreased again. The 800 °C-annealed samples in the N2 atmosphere showed higher visible light transmittance, however, the NIR shielding properties were lower than that of the non-annealed samples.

Moreover, the NIR shielding and heat insulation properties of CsxWO3 could be further improved after N2 annealing at appropriate temperature for a period of time. Particularly, the 500 °C-annealed CsxWO3 products in the N2 atmosphere showed the best NIR shielding and heat insulation properties. When the N2 annealing temperature was higher than 700 °C, the NIR shielding properties decreased again. The 800 °C-annealed samples in the N2 atmosphere showed higher visible light transmittance, however, the NIR shielding properties were lower than that of the non-annealed samples.

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |





 sales@chinatungsten.com
sales@chinatungsten.com