Improved Na2S Method Eliminating Molybdenum of Sodium Tungstate
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 18:30
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Recycling and Reusing Ammonium Paratungstate Crystal Liquor 1/3
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 18:28
(R3NH)6H2W12O40+6NaOH=Na6H2W12O40+6(R3NH)OH
Na6H2W12O40+18 NaOH=12NaWO4+10H2O
| APT Supplier: Chinatungsten Online www.ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nano-cemented Carbide Powder Synthesis
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 18:18
Before preparing nano-cemented carbide, the first thing is to synthesize the finer nano powder, which includes mechanical alloying (MA), spray conversion, senate situ carbon reduction and co-precipitation.
1. Mechanical Alloying (MA)
Mechanical alloying method is mainly through the use of mechanical driving force of high energy synthetic materials at low temperatures, high energy ball mill machine is one of the most commonly used tools. The research of nano-cemented carbide powder by MA has two main directions: one is use tungsten (W) and carbon (C) synthesizes to nano tungsten carbide (WC) powder by MA; another one is mixed tungsten carbide (WC) and cobalt (Co) powder, to meet the requirements of nano composite material by high-energy ball milling. Chinese scholars successfully W, C, Co mixed after milling synthesized nano scale WC-Co composite powder. It uses mechanical chemical method, which uses tungsten trioxide (WO3) and magnesium (Mg) and carbon (C) in nitrogen (N2) or hydrogen (H2) - Under an inert gas such as argon (Ar) atmosphere such as ball milling mixing occurs explosive reaction (after generation W and MgO, W and C diffusion reaction occurs, generating W2C and WC) prepared nano carbides WC, grain size is about 4-20nm. This method has simple operation, easy process and high efficiency, but the small granularity will be polluted by the friction between ball and the wall.
2. Spray conversion
Spray conversion process is also known as thermo-chemical method or fluidized bed process. Some scholars in related fields of the United States developed the method by metatungstate and cobalt chloride (CoCl2 • nH2O) solution or Co (en) 3WO4 and sulfuric acid (H2WO4) aqueous spray-drying and fluidized-bed reduction (flow bed reduction is a direct reduction process technology production of raw materials to produce tissue composition uniform nano powder (about 20-50nm) restore the furnace refractory composition structure) and carbonization reaction by constructing a fluidized bed reduction of iron ore.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Selective Precipitation Eliminating Molybdenum of Sodium Tungstate
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 18:10
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Methods of Eliminating Molybdenum from Sodium Tungstate Solution1/4
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 18:09
| Sodium tungstate Supplier: Chinatungsten Online tungsten-powder.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Concentration’s Effect on Ammonium Paratungstate Production
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 17:52

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate (APT) Nucleation Rate
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 17:19

| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Preparation of Tungsten Trioxide Thin Tilm 2/2
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 16:53
DC magnetron sputtering deposition of tungsten trioxide thin film on ITO conductive glass, oxygen partial pressure, sputtering power, composition and morphology influence electrochromic properties of temperature on single-layer structure of tungsten trioxide films. In order to optimize the performance of electrochromic thin films, monolayer film according to research results, based on a single-layer film of tungsten trioxide films were prepared by two-layer structure, studied film morphology of the films of the electrochromic properties of the composition. X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), atomic force microscopy (AFM), UV - visible spectrophotometer, electrochemical workstation variety of testing methods, the composition of the film, morphology, spectral and electrochemical properties were analyzed. The results show that tungsten trioxide films prepared under different sputtering conditions are non-stoichiometric ratio (O / W <3). The higher oxygen partial pressure, the tungsten trioxide films prepared O / W increases. When the oxygen partial pressure of 85% tungsten trioxide thin films having more excellent electrochromic properties.
Study results show that the sputtering power in the range of 50W ~ 100W, the sputtering power, density and roughness of the film increases. Appropriately increasing the sputtering power can improve the performance of electrochromic thin films. At room temperature, oxygen partial pressure of 85%, power 100W amorphous tungsten trioxide film microstructure when 120W prepared and have significant differences, and have good electrochromic properties. The maximum change of the transmission before and after coloring the tungsten trioxide films were 74% and 86%, coloring / bleaching response time was 9.6s / 2.9s and 9.3s / 3.9s, coloration efficiency were 45.07cm2 • C-1 and 43.11cm2 • C-1. Single nanometer tungsten trioxide film transmittance modulation capability and efficiency of the structure of the coloring preparation have reached a higher level to meet the requirements of adaptive camouflage.
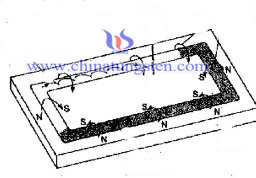
Trajectory magnetron sputtering target surface magnetic field and electron.
Tungsten trioxide film at 350 ℃ Sputtering is monoclinic state, improve the sputtering temperature lowered in the film O / W. After 200 cycles, the stability of crystalline tungsten oxide film may reach 99%. On the basis of a single-layer film structure study prepared three different structures and two-layer structure consisting of tungsten trioxide films, test results showed that: the film morphology and composition will directly affect the performance of electrochromic thin films. Bilayer sample preparation, the upper loose dense lower layer structure electrochromic thin film electrochromic best performance. Loose structure facilitates the diffusion of ions, to improve the response time of the film; a dense structure can improve ion storage capacity of the film. The kind of two-layer structure cycle stability tungsten trioxide films, the transmittance changes, coloring efficiency, coloring / bleaching response time was 84%, 74%, respectively, 19.86cm2 • C-1 and 64.6s / 99.7s.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Shielding Materials Application in Nuclear Plant
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 16:47

| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Preparation of Tungsten Trioxide Thin Film 1/2
- Details
- Category: Tungsten Information
- Published on Friday, 25 December 2015 16:34
By reactive magnetron sputtering and sol-gel method, tungsten powder peroxide polytungstate prepared thin films of tungsten trioxide. Tungstate peroxide polytungstate reactive magnetron sputtering method and prepare the necessary legal tungsten trioxide film Reagents, major equipment, preparation work, test procedures, and test flow chart lists the two methods, but also columns an orthogonal experimental factor level table and orthogonal design table. This paper using atomic force microscopy, dual-beam UV-Vis spectrophotometer, X- diffraction to characterize the tungsten trioxide film prepared by the above two methods.
AFM results show that: the sample surface tungsten trioxide film prepared by magnetron sputtering reaction is more uniform and compact than the surface of the sample prepared by sol-gel method, and the sample preparation tends plane molecular structure of the former, while the latter molecule sample preparation tends tetrahedral structure. Transmittance test results show that: the light transmittance of the sample are two ways to get closer, but the difference in absorption magnetron sputtering samples of different wavelengths of the sample is greater than the sol-gel method; transmission after annealing at 200 ℃ samples Spectral almost no change, but above 300 ℃ annealed sample transmittance decreased, and the decrease of annealing temperature, the higher the more the light transmittance.
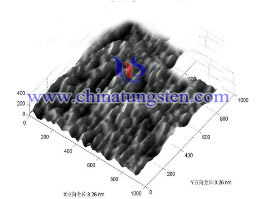
Sol-gel method sample annealed at 200 ℃ treated microstructure Figure
X- diffraction results show that: the sample after annealing at 350 ℃ was no significant spectrum sharp diffraction peak, indicating that all amorphous; samples after a temperature range of 350 ~ 400 ℃ annealing the resulting spectrum sharp diffraction peak intensity is growing, indicating gradually transformed into crystalline; the sample in the range of 450 ~ 500 ℃ annealed crystal diffraction peak intensity increases at the same time, the number also increased, because at this temperature for treating the sample gradually from a crystal into two crystal system coexist. Tungsten trioxide films prepared by these two methods are uniform and compact, good light, for the preparation of doped tungsten trioxide sensitive material to lay a solid foundation.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com