Cordierite Tungsten Trioxide Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Wednesday, 30 March 2016 14:51
 The development of the catalyst is the core technology in the flue gas denitration systems. It is a good choice to take cordierite as a carrier for fully uses the active ingredient inside the catalyst. Cordierite honeycomb ceramic is widely used in oil and chemical industry as the economic carrier because of its excellent properties, such as: thermal shock resistance, low expansion, wear resistance, well adsorption and high mechanical strength.
The development of the catalyst is the core technology in the flue gas denitration systems. It is a good choice to take cordierite as a carrier for fully uses the active ingredient inside the catalyst. Cordierite honeycomb ceramic is widely used in oil and chemical industry as the economic carrier because of its excellent properties, such as: thermal shock resistance, low expansion, wear resistance, well adsorption and high mechanical strength.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide SCR Flue Gas Denitration Catalyst Affecting Factors
- Details
- Category: Tungsten Information
- Published on Wednesday, 30 March 2016 14:48
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Surface Sedimentary Honeycomb WO3 Flue Gas Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 29 March 2016 18:29
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Mesoporous Foam Structure WO3 SCR Denitration Catalyst
- Details
- Category: Tungsten Information
- Published on Tuesday, 29 March 2016 18:27

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
W-Cu Thermal, Electrical Conductivity Comparison of Different Process (2/2)
- Details
- Category: Tungsten Information
- Published on Tuesday, 29 March 2016 16:59
While for tungsten copper (W-Cu) two phases heat sink material, it has lower coefficient of thermal expansion, which thermal expansion behavior is much more complex than a single-phase material. The experiment shows that t lower temperatures, tungsten-copper composite material showed a negative thermal expansion, but only when the temperature exceeds a certain value showed positive expansion. Tungsten copper sample coefficient of thermal expansion of injection molding and compression molding process under more stable than copper infiltration sample, the magnitude of change is smaller.
This is due to the phase change, as well as the internal organization of the reasons magnetic stretch, thermal expansion of the material will show some special law. By increasing the degree of constraint W phase at elevated temperatures in the expansion phase of Cu, thereby reducing the thermal expansion coefficient of tungsten copper composite material. In addition, since the difference of the coefficient of thermal expansion of the materials, tungsten copper composite material will produce complex stress inside, whose distribution will restrain the thermal expansion behavior.
As for the electrical conductivity, it was detected by eddy current method. When an alternating current is cut coil (also called probes) near the surface of a conductive material, since the coil alternating magnetic field, it has an effect on the material surface and near surface induced swirling current, which called the vortex. Materials and eddy currents generate their own magnetic field coil reacts, which is related to the size of the surface conductivity near the surface. Non-ferromagnetic conductive material can be directly detected by eddy current sensor. After testing found that the sample injection molded tungsten copper has the highest conductivity, reaches 37.43%IACS, which is higher than molding sample (29.85%IACS) and infiltrated sample (33.18%IACS).
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
W-Cu Thermal, Electrical Conductivity Comparison of Different Process (1/2)
- Details
- Category: Tungsten Information
- Published on Tuesday, 29 March 2016 16:55
Except test of hardness, density and micro-structure, for tungsten copper composite materials, which is widely used in EDM electrode, high-voltage discharge tube and heat sink, test of thermal properties (includes thermal conductivity and the coefficient of thermal expansion) and electrical conductivity are also essential. There is the test result of thermal and electrical conductivity by three different kinds of process:
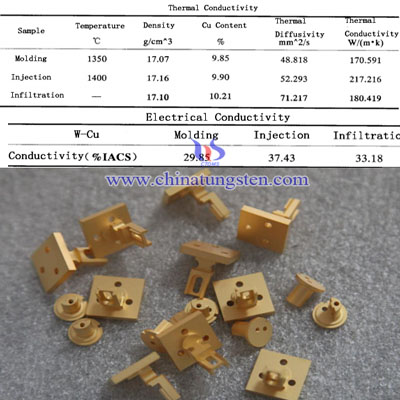
From the graph above, we can clearly see that among the three kinds of process, tungsten copper composite powder by PIM (Powder Injection Molding) has the highest thermal conductivity, reaches 217W/(m·k), compared with molding process and Cu infiltration. Combined with micro-structure of them, the researchers conclude some main reasons:
1. Although tungsten copper composite material has uniform distribution of W and Cu phase in the molding process, it still remains some porosity, which has a great impact on thermal conductivity of tungsten copper composite material;
2. Under the optimum Cu infiltration, there is no pore inside the sample, but Cu phase can not connect and form net structure. W and Cu phases distribute unevenly, in the process of heat conduction, part of thermal conductivity convey by W phase so that it is a critical factor of the lower thermal conductivity;
3. Relatively, injection molding process can effectively avoid the two defects, not only improve the density of tungsten copper products, but also W and Cu two-phase evenly distributed, thus it has a higher thermal conductivity.
Theoretically, thermal expansion of solid materials is due to the thermal vibrations of atoms as a center from its equilibrium position, which called crystal vibration non-harmonic effect. When the sintering temperature is increasing, atomic vibrations also stronger, the greater the energy of atomic vibrations, so that the microscopic atomic lattice parameters increase, the macro is manifested in the thermal expansion of solid materials. For single-phase material, the thermal expansion will increase as the temperature rises.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Cs0.33WO3 NIR Absorption Properties
- Details
- Category: Tungsten Information
- Published on Monday, 28 March 2016 17:20
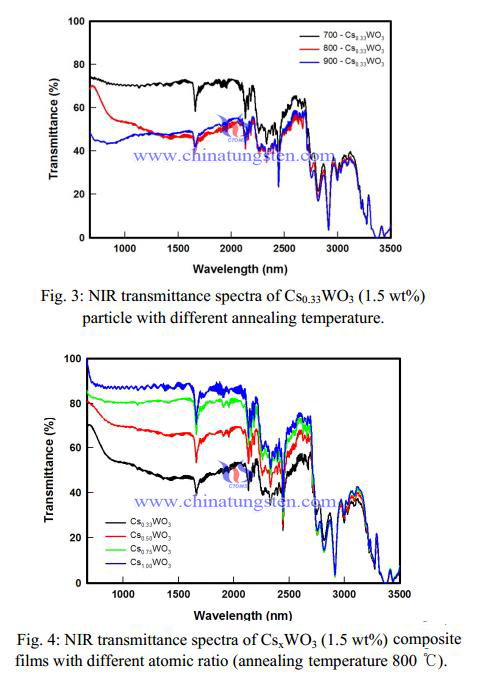
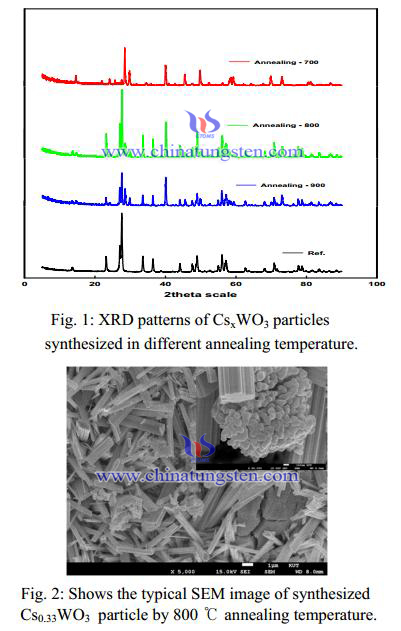
Figure 3 exhibits the properties of NIR absorption with different annealing temperature. These results indicated that the NIR absorption properties sensitively depended on the annealing temperature of Cs0.33WO3 particle. As above mentioned, it means that the effective absorption of NIR range for Cs0.33WO3 composite films increase with increasing content of hexagonal tungsten bronze structure of Cs0.33WO3 . This is consistent well with the XRD profiles of the samples shown in XRD patterns of CsxWO3 particles synthesized in different annealing temperature.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Annealing Temperature Effect on Synthesizing Cs0.33WO3
- Details
- Category: Tungsten Information
- Published on Monday, 28 March 2016 17:16
Figure 1 exhibits the typical XRD patterns of the synthesized Cs0.33WO3 particles with the different annealing temperatures. All peaks could be indexed to the hexagonal cesium tungsten bronze (JCPDS No. 831334) and the only Cs0.33WO3 particle synthesized in annealing at 800 ℃ almost was identified to disappear the impurity peak. It can be seen that the nanoparticles obtained by annealing at 800 ℃ prefers the hexagonal tungsten bronze structure. It was well known that the relatively larger ions such as Ti (0.150 nm), Rb (0.152 nm), and Cs (0.170 nm) compared with Li, Na etc. could fit better in the hexagonal vacant tunnels (0.163 nm) in the hexagonal tungsten bronze structure rather than in the rectangular vacant tunnels in the cubic tungsten bronze structure. In the other hand, this result suggested that the typical hexagonal structure of Cs0.33WO3 with remarkable absorption of NIR was formed by annealing at 800 ℃.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
CsxWO3 Powder Concentration Effect Experiment
- Details
- Category: Tungsten Information
- Published on Monday, 28 March 2016 17:12
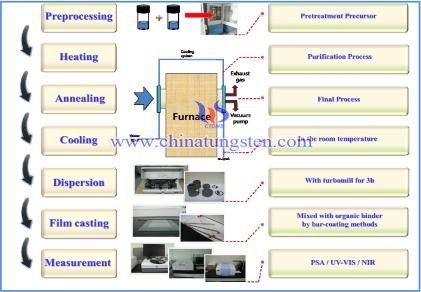
X-ray crystallography is a tool used for identifying the atomic and molecular structure of a crystal, in which the crystalline atoms cause a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their disorder and various other information.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Multiple Composite Rare Earth Tungsten Electrodes Production Technology
- Details
- Category: Tungsten Information
- Published on Monday, 28 March 2016 16:17

Multiple composite rare earth tungsten electrodes have good weldability, and non-radioactive, so it is the best alternative for thorium tungsten electrode. However, this electrode has low finished products rate and high production cost, thus greatly limiting its application range. Doping rare earth can help to reduce the work function of the electrode, improving the welding performance. However, the rare earth element will impede tungsten grain growth. And during processing, the rare earth will increase the recovery and recrystallization temperature of tungsten electrode, so that the deformation resistance of electrode is increased, making the sintering process not easy to control the, and finished products having low rate.
To improve multiple composite rare earth tungsten electrodes production technology will help improve the finished products rate and reduce production cost. To produce multiple composite rare earth tungsten electrodes using APT and rare earth nitrate as raw materials, doped with La, Y and Ce and other rare earth elements, can achieve uniform doping. Using two-stage reduction can produce good performance rare earth tungsten metal powder. During the restore process, you can use a large temperature gradient reduction method to control particle size. At sintering process, before the sintering neck grows up to closure making rare earth uniformly distributed in the tungsten bar, which can be obtained good performance sintered billet. In the process, appropriate increase processing temperature in the initial stage of the process, using multi-pass deformation processing technology, making the tungsten bar uniform deformation and improve processing rate and yield.
By strictly controlling multiple composite rare earth tungsten electrode manufacturing process technology can not only improve productivity, but also can obtain excellent performance tungsten electrode. In addition, it is also beneficial to composite rare earth tungsten electrode industrial production realization to reduce production costs and expand its application range.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com