Cesium Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:58
Introduction
Cesium tungsten oxide (CsxWO3) is a kind of functional compound which has nonstoichiometry ratio and oxygen octahedral, it has low electrical resistivity and low temperature superconducting property. CsxWO3 is discovered to have great near infrared shielding property which is expected to take replace of ITO as raw material for smart window, it has wide application prospect in automobile and architecture field.

Preparation
Citric acid induction thermal synthesis method
According to chemical formula CsxWO3(0<x<0.33), valance state of W is at reduction state. With the increasing of Cs ion, low valance state of W ion also increases. It means synthesis of CsxWO3 needs a certain reduction atmosphere.
During this process, citric acid and ethanol is used as reducing agent, carboxyl and hydroxyl will be oxidation into carbon dioxide under high temperature and pressure. And W6+ can be reduced into W5+ and W4+. Cs ion added into tungsten bronze which the process is like following:
1. Preparation of CsxWO3 powder
1) Use AR grade sodium tungstate Na2WO4·2H2O as raw material, configurating sodium tungstate solution for concentration of 0.5 mol/L;
2) Obtain tungstic acid solution by ion exchange resin method, adding citric acid solution of concentration 1mol/L or 2 mol/L and 0.3mol/L Cs2CO3 solution, stick it evenly, the precursor solution is obtained;
3) Adding precursor solution into autoclave, reacting for 3days under 90℃, after ultrasonic washing, alcohol washing, centrifugal and dry the CSxWO3 is obtained.
2. Preparation of CsxWO3 thin film
Grinding CsxWO3 powder, ultrasonic dispersing it in citric acid solution for 1hour, then add into citric acid solution for ultrasonic dispersing for 1h, then adding 0.1g/ml PVA solution, stirring it for 30min then aging 1day to get latex. Use sol-gcl dip-coating method to prepare CsxWO3 thin film on glass slide.
Property and application:
Near infrared shielding property and heat insulating property
Near infrared shielding material often refers to a kind of functional thin film material which has strong absorbance or reflect ability of near infrared and won’t affect its visible light transmission. As transparent insulating material, it has wide application prospect in architecture and automobile insulating glass.
CsxWO3 thin film can shield near infrared for wave length longer than 1100nm. The near infrared shielding property and heat insulating property of glass after coated with CsxWO3 thin film increases with the Cs content in CsxWO3. Compared to common glass, the insulating temperature difference can reach 13.5℃.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Hydrogen Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:53
Hydrogen tungsten bronze(HxWO3,0≤x≤1) is non stoichiometric compound, it has ring channel and special space tunnel structure. This structure is useful for disembedding and exchange for ion, thus makes it capable of offering and accepting proton.
Proton exchange membrane fuel cell (PEMFC) has advantages of low reacting temperature, high power density, high efficiency and no pollution. It is being widely used in portable power supply, dynamical power and power station. Traditional catalyst uses Pt as main content, which is quite expensive. So adding auxiliary catalyst is an effective method to lower the using amount of Pt and promote its catalytic activity. Hydrogen tungsten bronze as auxiliary catalyst to combine with Pt, the proton will accelerate the oxygen reduction of Pt by offering, by accepting proton can accelerate the oxygen catalytic of Pt to methyl alcohol. As a result, it has wide application broad as PEMFC.

Preparation
1) APT (NH4)10(H2W12O42)7H2O is decomposed to (NH4 )10(W12O41)5H2O under heating to 100 ~200 ℃.
2) (NH4)10(W12O41)5H2O keeps decomposing to (NH4)0.33WO3 under 200-250 ℃.
3) Under 250-575 ℃, (NH4)0.33WO3 converts into H0.33WO3 and WO3.
Among which (NH4)xWO3 can be decomposed into HxWO3 under 150-350 ℃ with change in crystalline structure, chemical equation is: (NH4)xWO3=HxWO3+xNH3(g).
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:47
Composition and structure
Crystalline structure of NaxWO3 is affected by value of x. When x<0.1, NaxWO3 is WO3 structure, it uses WO6 octahedral as unit structure. When 0.1 <x <0.35, NaxWO3 is tetragonal crystalline. When 0.35 <x <1, Nax WO3 has perovskite structure with Na+ vacancy. These two crystalline structure has WO6 octahedral structure unit. Under different temperature, WO6 octahedral can flex in different directions to make the temperature change of crystalline structure. It offers the possibility of synthesis for different materials.
Lattice imperfection
NaxWO3 equals to NaWO3 without (1-x) Na+. NaWO3 belongs to complete perovskite structure which is cubic system. There is no space in lattice for they are being taken by Na+、W(v),O2-. When NaWO3 loses Na+ and becomes NaxWO3, lattice appears (1-x) Na+ space, Na+ space made the incomplete of lattice, that’s why NaxWO3 appears single ion default.
Synthesis
X value of NaxWO3 is determined by synthesis condition. Under high temperature, Na+ would disperse into lattice or spread out from it, it makes the content of sodium changes in NaxWO3. Using mixture of sodium tungstate and tungsten oxide and heat them with proper reductant would get the product, the reductant is often W、WO2、H2. Also, by electrolysis of sodium tungstate and tungsten oxide can also obtain the product. Chemical equation is as following:
3xNa2WO4 + (6-4x) WO3 +xW=6NaxWO3
xNa2WO4 + (2 -2x) WO3 +xWO2 =2NaxWO3
xNa2WO4 + (2 -x) WO3=2NaxWO3 +x/2O2
Property
Close packed structure, chemical inertness and metal luster
NaxWO3 belongs to fake and default ABO3 perovskite structure. In such structure, O2- and Na+ are in cubic close packing, W and O becoming WO6 octahedron and share the same vertex, Na+ lies in the interspace of WO6 octahedron. So NaxWO3 can resist all kinds of hydrofluoric acid, insoluble in water, shows ultimate chemical inertness to acid. Its close packed structure makes it have metal luster and inert metal property. As a result sodium tungsten bronze can be used as great anti-corrosion material.
Unstable oxidation state and reduction of W in NaxWO3
Average oxidation number of W in NaxWO3 is between V-VI, xmol of W is +V oxidation state, but the most stable is +VI, it makes NaxWO3 have strong reducing property under alkali condition. NaxWO3 is oxidized when heating in the air, it can be soluble in the strong alkali solution, and can reducing ammonium solution of nitrate.
Color of NaxWO3 changes with the x value. The reason why crystalline has coloration is there is default in it which is called color center. Different x value of NaxWO3 would cause difference in absorbing visible light wavelength, thus the crystalline color is different. When x value is bigger, electron transition mainly absorbs short wave blue-purple light, the crystalline would show yellow-orange; as the decrease of x value, electron transition absorbs long wave red-orange light,
Electrical conductivity
Sodium tungsten bronze has electrical conductivity which is related to its composition. When x>0.25, NaxWO3 shows metallic electrical conductivity, rate decreases with rising temperature. When x<0.25, NaxWO3 shows semiconductor metallic electrical conductivity, rate increases with rising temperature. This special property makes it become newly solid electrolyte, there is already NaxWO3 used as ion reversible electrode. Synthesis and research on solid electrolyte is an active field of inorganic solid chemistry. Solid electrolyte replaces regular electrolyte solution has become revolution of electrical chemistry. The crystalline shows blue-purple.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nano Cesium Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:42
A large number of transparent materials like glass, plastic are used in modern building, while these materials can improve indoor lighting, they will also lead to the sun light going into the room thus cause indoor temperature rising. In summer, people often use air conditioning to cool down the room temperature, this is the main reason for powder cutting in the summer in China. With the growing popularity of automobiles, the insulation film has become the standard configuration of a car for cooling down temperature inside the car. Nowadays the most effective way is adding nano particles which has near infrared ability into resin such as ATO, ITO and nano cesium tungsten bronze powder to preparing transparent heat insulating coating which can be coated on glass or Havelock, or coated on PET thin film in advance, then sticking it on the glass. It can also be made into plastic thin film, like PVB, EVA plastic, then recombine it with tempered glass to resist infrared ray. Among these nano particles, cesium tungsten bronze has the best near infrared shielding property. Usually adding 2g into per square meter of glass can reach transmittance less than 10% at 950nm(absorbance for near infrared ray). At the same time, at 550nm can reach transmittance more than 70%(70% refers to the index of mostly high transparent thin film).

Preparation method:
Solvent hot liquid phase method preparing nano cesium tungsten bronze:
1. Heating and dissolving 400kgs sorbitol in jacket reactor, adding tungstic acid and cesium sulfide, its mass ratio is 1:0.33. Mass of sorbitol is three times more than total weight of tungstic acid and cesium sulfide.
2. Stirring 30min under high speed, homogenate it into homogenizer, after 60min, adding the obtained material into autoclave which is heated to 150℃. Set the rolling rate of autoclave to 180r/min, after the above material is totally converted into autoclave, turn off the valves of it and heating it to 350℃, stay it warm for 600min, then lowering the temperature to 150℃.
3. Adding deionized water into reaction material, put them into press leaching machine, washing them with deionized water and absolute ethyl alcohol until sulfate radical content less than 100mg/kg, ethyl alcohol content more than 80%.
4. Dry the leaching material in vacuum drying machine, then processing mechanical pulverization and jet mill, the dark blue cesium tungsten bronze nano powder is obtained.
Cesium tungsten bronze nano sizing agent and transparent heating insulating coating preparation method:
1. Adding cesium tungsten bronze nano powder, deionized water, dispersant, sodium hydroxide or nitric acid into stirred tank, after stirring, put them into sander and then grind them to disperse. Until the particle size of sizing agent stays stable, stop grinding, the cesium tungsten bronze nano sizing agent is obtained.
2. Adding the sizing agent into acrylic emulsion, coating it on the glass substrate, then dry it to get the thin film of 5um, the adding amount is 1.3g/m2.
Using xenon lamp to keep radiate the cesium tungsten bronze transparent heat insulating thin film, after 72hous, its appearance didn’t change obviously, it proves that cesium tungsten bronze nano powder has good resistance. The mechanism for it to shield infrared ray is the absorbance of infrared ray by oxygen valance in the nano powder. Oxygen valance would react with moisture in the air to lower its concentration, so the insulating effect is lower down, Soaking the insulating thin film in 60℃ hot water for 168hours, the shielding rate only drops for 1.8%, it shows the cesium tungsten bronze nano powder has better moisture resistance. The service life is approximately 20 years which can largely lower the cost of thin film.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Potassium Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:39
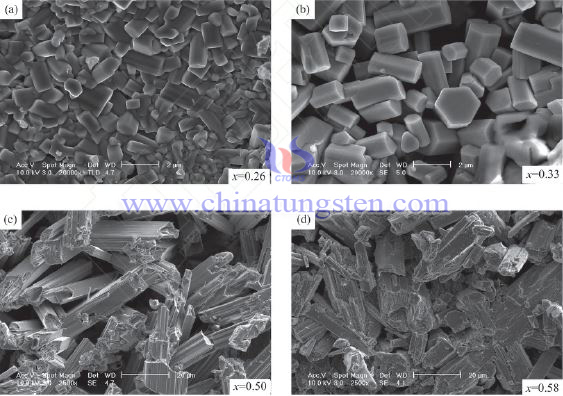
Potassium tungsten bronze(KxWO3) has crystalline structure for hexagonal (0.18≤x≤0.33)and tetragonal(0.40≤x≤0.59), it has super conductive property which shows charge density waves. Color of KxWO3 changes with value of x. When x increases, it turns from dark blue(x=0.20) to purple(x=0.60).
Below is the SEM for KxWO3 when x=0.26,0.33,0.50,0.58:
Preparation:
1.Mix K2WO4(dehydrate K2WO4·2H2O under 200℃ for 2 hours) and WO3 for mole rate 1:7, weigh 2g and mix them up.
2.Configurate stainless ball mill (Ø6 mm) by weight rate 20:1, put mixing material into stainless steel milling container, sealing with cap and draw out the air, then inflating with Ar.. Mill it on planetary ball mill for 10h (rotary speed 450r/min).
3. Press and cut the material under 20MPa, then put it into quartz tube, vacuumizing and then packing.
4.Put quartz tube into high temperature rotary and heat to 800 ℃ and 750 ℃, cool it down to room temperature.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:28
Ammonium tungsten bronze(ATB) is a kind of blue oxidation material with hexagonal or tetragonal structure which contains ammonium. If is more active than blue tungsten oxide, especially ammonium ion can interexchange with doping element—potassium. During reduction process, it will form tungsten phase structure with potassium which is helpful for potassium enters tungsten and disperse.

Preparing method:
1.Adding 0.01~1g organic tungsten source to dissolve in 20~40ml organic acid solution, and stir it.
2.Then adding 4~30ml organic ammonium, mix them up, remove to reaction still, hydrothermal crystallization for 0.5~48hours.
3.Contrifuge and wash the powder sample after reaction, and dry it under 40~250℃ vacuum circumstance for 1~12hours, the nano ammonium tungsten bronze particle is obtained.
By this method, hexagonal ammonium tungsten bronze can be obtained, its grain size can be adjusted between 80~500nm, of which the appearance is uniform, and has narrow particle size distribution. The chemical valance state exists as W6+ and W5+, full of free electron. Apart from that, ammonium tungsten bronze has strong absorbing infrared ray. Thin film contains nano particles can shield 780~2500nm of near infrared ray and can keep high transmittance,.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 14:18
Introduction
The tungsten bronzes are a group of compounds made up of tungsten trioxide, WO3, and an alkali metal, such as sodium (Na), potassium (K), rubidium (Rb), or cesium (Cs). The general chemical form is MxW03, where M=Na, K, Rb, or Cs, and 0<x<1. The color of these compounds varies with composition, at x=0.93 the color is a bronzelike golden-yellow; at x=0.32 the color is blue violet.
The variation in composition also affects the structure of the compound. Imagine a cube with a tungsten atom at each corner, an oxygen atom in the middle of each edge and an atom of an alkali metal in the center of the cube. However, in a tungsten bronze there is not an atom at the center of every cube. When x<1, only a certain fraction of the cubes will contain an alkali atom. If x is large, close to 1, the structure of the crystal lattice will be cubic. As x decreases, fewer of the cubes are filled, the structure changes. At about x<0.3, or with less than 30% of the cubes full, the structure becomes hexagonal, with atoms arranged in hexagonal plates.
The cubic arrangement described above with an atom in the center of a cube is typical for perovskites, a group of ceramic materials with a variety of interesting electrical properties. The high temperature superconductors are among these. In the cubic phase, tungsten bronzes are metallic and conduct electricity. However, in the hexagonal phase, they become superconducting.
Classification
Tungsten bronze has special space tunnel structure, classified by crystalline structure, it can be divided into perovskite tungsten bronze (PTB), tetragonal tungsten bronze(TTB), hexagonal tungsten bronze(HTB) and intergrowth tungsten bronze(ITB). PTB and HTB belong to special non-stoichiometricm tungsten bronze can be divided into the same category as non-stoichiometricmn compound. W exists as W6+, W5+ and W4+ in tungsten bronze to balance the electrical charge of compound. Tunnel structure and its special valence state makes it have superior electrical and ion conducting property, superconducting and photochromic property. Its application in storage battery, electrochromism and chemical sensor has draw great attention.
1. Non-stoichiometricmn compound
PTB and HTB are special non-stoichiometricmn tungsten bronze, its chemical formula is MxWO3(0< x < 1 ), M can be alkali metal like K, Na, or Ca、Sr、Ba and other rare earth metal like Cu, Ag, H. They have high electrical conducting property and fast ion transport property, its electrical conducting rate can reach 2.5X106 S/m which belongs to low temperature super conductor.
2. Intergrowth tungsten bronze
ITB contains B2O62- is due to lack of O or the extra ion B, or BO takes the biggest vacancy of the compound. Usually, it can be expressed as(AO)m.(B2O5)n or(AO)m.(BO3)n according to its valence state. BaO. The main products are(Nb2O5)2、BaO.(Ta2O5)2、Nb8W9O47.
3. Tetragonal tungsten bronze
TTB is the mostly seen and used in the tungsten bronze structure, it has become the focus of research and development. Many orthogonality tungsten bronze is the super structure of TTB, so it is classified into TTB.
Preparation
1. Wet chemical method
Wet chemical method is firstly applied in the preparation of HxWO3. The common wet chemical method preparation method contains the steps of: Soaking WO3 crystalline in acid and metal power(Zn, Pb, Sn) solution, react in special container(such as Jones reactor, can realize separating between reactant and air). During processing, H enters into vacancy of WO3. For example, H0.3WO3 of hexagonal structure can be prepared by hexagonal WO3, hydrochloric acid and Zn.
Precursor compound decomposes in solution can obtain tungsten bronze. For example, APT (NH4)10(W12O41) 5H2O mixed with solution like glacial acetic acid and heating to 200 ℃,hexagonal structure (NH4)xWO3 can be obtained.
Due to low temperature during synthesis process, the crystalline state of product is better so the method has become the focus of research.
2. Thermal reduction method
2.1 Phase-phase heating reduction method
Below is the preparing process: Mix WO3, tungsten metal powder or WO2 and tungstate of metal M, heat it to 1000℃ under vacuum atmosphere or inert atmosphere. Then remove the impurities to get MxWO3. Reaction equation is :
x/2M2WO4 + (1-x)WO3 + x/2WO2→MxWO3
2.2 Thermal decomposition method
Heating precursor compound like HPAs complex, peroxy-HPAs complex and other complex compound containing tungsten can decompose tungsten bronze. The thermal decomposition method of APT to obtain hydrogen tungsten bronze contains following steps:
1) Heating APT: (NH4)10(H2W12O42)7H2O under 100 ~200 ℃ to obtain (NH4)10(W12O41)5H2O;
2) (NH4)10(W12O41)5H2O is continued to decompose into (NH4)0.33WO3 under 200-250 ℃;
3) Under 250-575 ℃, (NH4)0.33WO3 shows great stability, it turns into H0.33WO3 and WO3.
Among which (NH4 )xWO3 can be decomposed into HxWO3 under 150-350 ℃ with change in crystalline structure, chemical equation is: (NH4)xWO3=HxWO3+xNH3(g).
3. Electrochemical method
Electrochemical method is the common used in the preparation of hydrogen tungsten bronze and lithium tungsten bronze. Its producing process contains: converting WO3 into electrode, then using WO3 electrode as cathode, using graphite or Pt as anode, electrolysis in sulfide acid or nitric acid can get hydrogen tungsten bronze or lithium tungsten bronze, the chemical equation is as following:
xH++WO3+xe=HxWO3
xLi++WO3+xe=LixWO3
Property and application
Tungsten bronze has good electronic and ionic conductivity, superconductivity and optical properties, which has broad application prospects. In these performance of tungsten bronze, the conductivity and superconductivity have been researched earlier, especially the superconducting properties, which had become the focus in the sixties and seventies. Although there are reports about tungsten bronze type conductivity and electron conduction now, the conductivity and superconductivity is no longer the main content of performance studies.
Tungsten bronze was formed after counterion embedded in WO3 under certain conditions, and it has colors itself because of the absorption and scattering of light, and the intensity of the absorption and scattering change with the x value changing, which also makes tungsten bronze present different colors. Optical properties of tungsten bronze are more prominent because of plasma H +, Li +, Na + and Ag + embedding, of which there’re more researches. In which HxWO3 has strongest absorption of light, Li + followed. However, lithium and sodium tungsten bronze have maximum light absorption when x ≈ 0.6, while the hydrogen tungsten bronze does not.
Electrochromism device and photochromism device as two important aspects of the tungsten bronze applications, optical performance is one of its basic principles. Electrochromism applications are related to plasma H+ and Li+ electrochemical reversible embedding on WO3 electrode.
Optical embedding is that plasma H+, Li+ and Na+ into WO3 reversibly solid under irradiation of light, which is also an important performance of tungsten bronze, and it is the basic reactions to achieve color light system, photoelectric conversion and other applications. Because it’s the mixture of WO3 and tungsten bronze after intervention, this effect was also seen as an optical doping process.
New performance of humidity sensitive properties to the sensitive nature of some chemicals may also makes tungsten bronze effective application in humidity measuring instruments, chemical sensors and other devices.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Crucible for Quartz Continuous Melting Furnace
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 11:57
Quartz glass is a kind of special glass only made of silica, whose microstructure is a simple network which is made of tetrahedral structure of silica, having a compact structure. The optical property of transparent quartz glass is excellent. Except that, it also has an excellent transmittance in the range of continuous wave between ultraviolet and infrared radiation. Quartz glass formed by quartz continuous melting furnace can be widely applied in various processes of the semiconductor manufacturing.
Tungsten crucible has a high melting point, high boiling point with an excellent high temperature strength property, therefore, it is widely used in quartz continuous melting furnace. At present, the main process equipment is installing the molybdenum electrodes and 64molybdenum rods in heating device of quartz continuous melting furnace, these molybdenum electrodes and molybdenum rods would spread evenly around the wolfram crucible. When the electrodes are conducted by electricity and heating, as the conductive heating element, 64 molybdenum rods would make wolfram crucible continue heat up.

However, this process equipment has a serious flaw. When these 64 molybdenum rods are considered as the conductive heating element, there exist an 8-12mm distance between molybdenum rods which can cause heating of the wolfram crucible unevenly, thereby prolonging its heating time, it would greatly reduce the production effectiveness of quartz continuous melting furnace. In order to deal with this problem, users can install charge pipe and tungsten core rod on the top wolfram crucible of the furnace to protect the entrance of the gases. Putting a molding material platform whose central section should be designed with a forming port, and then put the heating unit on wolfram crucible external. Molybdenum rods can be replaced by molybdenum network and coverage the surface of wolfram crucible evenly. The uniform coverage of molybdenum network can make tungsten crucible be heating evenly, so that it can effectively solve the quality problems.
In addition to quartz continuous melting furnace, tungsten also plays an important role in powder metallurgy technology, electronic spraying, and crystal growth. The production of quartz glass requires the high purity quartz sand continue to flow into the tungsten tube of continuous furnace, the material continued to be melted at a temperature above 2000 ° C, and then flow continuously along the tungsten material table, finally, freeze it. Most of the materials would become deformation under the continuous high temperature, while the wolfram crucible can withstand the test of the process.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
How to Detect Internal Defects of Tungsten Crucible?
- Details
- Category: Tungsten Information
- Published on Friday, 29 April 2016 11:54

Due to the different maturity of manufacturing process for tungsten crucible, there are some potential problems of the finished products which are difficult to find. Wolfram crucible is mainly formed by the tungsten powder molding which requires a lot of pressure during the pressing process. With the increase of inner stress of tungsten product, the inside of wolfram crucible would crack. Therefore, after the manufacturing process of the wolfram crucible, inside of it should be carefully examined. The followings will give a brief introduction of the steps for detecting internal defects of wolfram crucible.
Phased array ultrasonic testing equipment is the essential tool for detecting internal defects of wolfram crucible which is divided into three parts, choosing the best configuration of phased array ultrasonic testing equipment to test them respectively by ultrasonic detecting. It takes a short time to finish the examination only for10 minutes, and the operation is not complicated, with a high effect that can be applied to large-scale wolfram crucible producing.
Since wall of wolfram crucible is thin with a large area, it can be detected by double crystal probe in phased array ultrasonic testing. The structure of wolfram crucible bottom is relatively simple with a smooth surface, so inspector can use a high-frequency linear probe to detect it. The structure of the junction between wolfram crucible wall and bottom of the crucible is complex, deformation surface is relatively large, so inspector can use high sensitivity test block for its detection which can detect the smallest equivalent of 0.2mm hole.
Because of the particularity of the manufacturing process of the wolfram crucible, it would cause instable quality of wolfram crucible. The inside of wolfram crucible is easy to have cracks, holes and other defects. If it cannot be noticed in time which would cause further more severe crack during application or lead to a wolfram crucible burst, even result in serious accidents. Therefore, the detection of internal defects of finished wolfram crucible is necessary. Detecting by this method can ensure quality of product, and the detection result of the method is more intuitive, the cost of the process is low and it’s suitable for large-scale industrial production of wolfram crucible. Quality of wolfram crucible directly determines the quality of the follow-up work. Development of testing technology for nondestructive wolfram crucible has a great significance for industry. People should think highly of its applications.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Crucible for Crystal Sapphire Growth
- Details
- Category: Tungsten Information
- Published on Thursday, 28 April 2016 18:39
Single crystal sapphire is playing an ever-increasingly important role as a vector for high reliability electronics today which belongs to synthetic crystal. Thanks to a good optical permeability, thermal conductivity and mechanical properties, single crystal sapphire is mainly applied to wear resistant elements, window materials and electronics devices. With the rapid development of science and technology, the demand for single crystal sapphire is rising. As a necessary vector for single crystal sapphire growth, the quality of tungsten crucible will directly determine the outcome of sapphire crystal growth.
Manufacturing technologies for sapphire crystal include Flame Fusion, Czochralski, Heat Exchanger method, Kyropoulos, Bridgman-Stockbarge method, Edge Defined Film, Vertical Horizontal Gradient Freezing.
Czochralski: First of all, put the polycrystalline material which has been prepared before in tungsten crucible, heat them to above 2050 ℃ and the raw material will be melted into flux. There are some seed crystals under the lift rod of the tungsten crucible. Reducing the lift rod to make seed crystals insert flux, at an appropriate temperature circumstances, and the seed is in a fixed state then pulling up it in a certain rate. Heat exchanger method is to put seed crystals at the bottom of the tungsten crucible, through controlling the flow of helium gas of the tungsten crucible bottom to ensure that seed can keep in a cold condition. After all the tungsten crucible material being melted, increase the flow of helium gas, so that the solid liquid interface moves upward.
Bridgman-Stockbarge method is to put a seed in bottom of tungsten crucible, after all the raw materials being melted, welding the seed crystal and the melt. And then crystal would obtain a temperature gradient through moving the high temperature zone and low temperature zone of tungsten crucible. Finally, solid-liquid interface move upward to finish sapphire crystal growth.
Tungsten crucible applied in sapphire single crystal growth furnace can avoid sticking the pan when sapphire crystal growth. The internal wall and external wall of tungsten crucible are smooth without any crack which can greatly improve the efficiency sapphire crystal growth.
On the other hand, due to the high melting point 3410 ℃of tungsten, the tungsten crucible is widely used as the core container in sapphire growth furnace, quartz glass melting furnace, rare earth smelting furnace and other industrial furnace.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |




 sales@chinatungsten.com
sales@chinatungsten.com