Tungsten Boat for Vacuum Coating
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 17:54
Thanks to a high melting point, a low vapor pressure and stable chemical property, tungsten boat is widely used in vacuum evaporation coating industry as a resistance evaporation source.
Vacuum coating technique is a kind of technology to produce thin film material by physical means. In the vacuum chamber, atomic of material is isolated from the heating source and hit the plated object’s surface. This technology was first used in the production of optical lenses, such as navigation telescope lens. Later it extended to other functional film, such as music aluminizing, decorative coating and surface modification. Vacuum coating includes three types, named vapor deposition, sputtering deposition and ion plating.
As a main source of resistance heating, W (wolfram) boat is widely applied to vacuum deposition: use a refractory metal, such as W-boat, to form the shape of boat or shape of wire, i.e. Make the current pass them, and then heat evaporated material which place above it or place in the tungsten crucible. Adopting W-boat for vacuum deposition would save more material which can accelerate the speed of volatilization of powder, the required temperature. On the other hand, it just need low temperature and heating current but evaporation brilliant is much stronger which can produce film on the substrate.
Tungsten boat for vacuum deposition can make the material surface metallization, combine the organic materials and inorganic materials to further improve its physical and chemical properties. In addition, it can also improve the surface hardness. Vacuum deposition can increase its hardness and abrasion performance. And reduce the absorption rate, the more the coating times, the less vacuum, and the absorption rate will be reduced. Products would not easily to be deformed, and its heat resistance would enhance. Vacuum deposition has a high deposition rate if using wolfram boat.
When use W-boat for vacuum deposition, operators should keep inflammable poison safely in case of fire poisoning. When washing parts and wolfram boat, operators should wear rubber gloves. When off duty, don’t forget to turn off the power and disconnect water source.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Notices of Tungsten Boat for Vacuum Coating
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 17:51
 During vacuum coating, people often do not pay attention to some details which would lead to an unsuccessful coating. Compared to other industries, vacuum deposition is a very environmentally friendly technology for its lower electromagnetic radiation. In addition, it doesn’t have great impact on human bodies. Therefore, the operator should work in more details to improve the efficiency of vacuum deposition.
During vacuum coating, people often do not pay attention to some details which would lead to an unsuccessful coating. Compared to other industries, vacuum deposition is a very environmentally friendly technology for its lower electromagnetic radiation. In addition, it doesn’t have great impact on human bodies. Therefore, the operator should work in more details to improve the efficiency of vacuum deposition.
The first is to pay more attention to the maintenance of working environment of vacuum deposition. Make sure that the substrate surface of it was cleaned before. To reach purpose of deoiling, decontamination and dewatering of the workpiece. In the process of machining a workpiece, transport and packaging, various dusts, lubricants, polishing paste, oil, sweat, etc. would adhere to the substrate surface. Under humid conditions, the materials on surface will form an oxide film. If this happens, it can be removed through deoiling or chemically cleaning method. If there is no surface cleaning treatment, it cannot be placed in the atmosphere. Users should use a closed container or cleaning cabinet to keep it which can reduce dust pollution. For the highly unstable and sensitive to water vapor surface, it is better to be stored in a vacuum oven.
In addition to the maintenance of the environment, operators should regulate their operations. At the time of opening the vacuum deposition machine, operators should turn on the water carrier firstly and observe the water pressure uninterruptedly. When the ion bombardment and evaporation, high voltage cable cannot be touched in case of electric shock. In order to prevent human body from X rays, operators would better wear the lead glass during the observation. During plating the dielectric multilayer film coating, ventilated vacuum devices should be installed to remove harmful dust in time. After working, don’t forget to turn off the power and disconnect water source. In the whole process, operators must wear full protection and cleaning sheath.
Tungsten boat is important resistive evaporation source for vacuum deposition, and due to the different types of it, it is better to choose the corresponding type to coat. More importantly, tungsten boat's surface cannot have any cracks, keeping smooth and flat. Provided that there is some trouble of it which should be changed to a new one immediately. Clearing the dust of the coating indoor, setting the high cleanliness of the workplace, keeping the high cleanliness are the basic requirements of the coating process on the environment.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Prepares Hydrodesulfurization Catalyst
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 17:25
 Currently, the widely used of liquid fuel hydrodesulfurization catalysts in industry are mostly supported tungsten sulfide (or molybdenum) based catalysts which adds nickel, cobalt and other aid. Such catalysts usually take ammonium tungstate or ammonium molybdate as raw material, and obtained after impregnation, pyrolysis. The particle size, degree of dispersion of the active ingredient in tungsten oxide containing desulfurization catalyst cannot achieve the desired state. In this situation, developing a higher activity catalyst with highly dispersion and nanoscale has become the main research issue of the scientists.
Currently, the widely used of liquid fuel hydrodesulfurization catalysts in industry are mostly supported tungsten sulfide (or molybdenum) based catalysts which adds nickel, cobalt and other aid. Such catalysts usually take ammonium tungstate or ammonium molybdate as raw material, and obtained after impregnation, pyrolysis. The particle size, degree of dispersion of the active ingredient in tungsten oxide containing desulfurization catalyst cannot achieve the desired state. In this situation, developing a higher activity catalyst with highly dispersion and nanoscale has become the main research issue of the scientists.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Boehmite Prepares WO3 Contained Hydrodesulfurization Catalyst
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 17:22
 Boehmite is the main raw material to produce aluminum oxide. γ-Al2O3 obtained by the calcination of boehmite as the carrier, and load nickel oxide and tungsten trioxide, add a additive-fluorine, thus to produce tungsten trioxide hydrodesulfurization catalyst with high activity. The different purities of boehmite have a certain degree of influence on the activity of the desulfurization catalyst.
Boehmite is the main raw material to produce aluminum oxide. γ-Al2O3 obtained by the calcination of boehmite as the carrier, and load nickel oxide and tungsten trioxide, add a additive-fluorine, thus to produce tungsten trioxide hydrodesulfurization catalyst with high activity. The different purities of boehmite have a certain degree of influence on the activity of the desulfurization catalyst.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Button Distribution Optimization on Drills (2/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 16:50
In the early 1980s, some researchers have proposed the distribution of the drill bit buttons should follow three guidelines: 1. each cutting button has equal rock breaking volume; 2. equal wear rate; 3. equal rock consumption. Meet these three criteria in order to ensure rock breaking energy evenly distributed on each cutting tooth, so as to obtain the best rock-breaking effect. Theoretically, the process of tungsten carbide buttons for rock breaking can be divided in to three steps: elastic deformation (when the force is released, the rock surface can restore the status), fatigue process (also known as pressure crushed stage, surface cracks do not disappear and fracture rock surface) and volume breaking (The formation of shear body, under increasing pressure formed pit crushing).
When the adjacent tungsten carbide buttons impact the rock at the same time, the overall crushing effect depends on the nature of the deformation of the cross-belt. Reasonable button spacing will produce elastic deformation energy to rupture and push the rocks out with the increasing load; if the spacing of two buttons is too narrow, it will make the compacting zone too close to increase the difficulty of the shear rock; if the spacing of two buttons is too wide, the rock in the middle part will not be cracked and form rock ridges. Viewed from the impact energy, for the drills fixed diameter, the less number of buttons, the more impact energy of each button endure and the rock crushing volume will also increase accordingly. However, if the number of button is too small, which over the stress fracture of a single button, tungsten carbide button will be broken.
Buttons distribution firstly depends on the shape of the bit end face, which includes flat-shaped, arc-shaped, protruding, concave shape, and so on. The most used are flat-shaped and arc-shaped. Generally, tungsten carbide drill bit with large diameter usually uses arc end face and the small diameter often uses the flat-shaped. In intermediate buttons distributed in the cross section perpendicular to the bit axis, while the button are located on the inclined face of the drill bit. The outermost edge of buttons not only has to be mechanical support, but also should withstand the crushing function so that it has a greater amount of consumption than the buttons middle. Furthermore, it has high speed of linear velocity and requires a larger diameter, wider distribution and excellent quality.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Button Distribution Optimization on Drills (1/2)
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 16:49
The main component of tungsten carbide button is the hard phase of WC and the binder phase of Co, which has high hardness, high strength, excellent wear resistance and corrosive resistance and perfect impact resistance. So it can be widely used in high-pressure DTH (drill-the-hole), oil drilling, tunneling, mining industry and civil architecture. Due to its excellent wear resistance and impact resistance, compared with other materials, and it has higher drilling speed and the life span of drills and bits will be extended so that it does not need to change buttons constantly, which improves the overall efficiency and reliefs the labor.
On the basis of ensuring the stable properties of tungsten carbide buttons, the quantity and the distribution of buttons on drills and bits are also the significant factor on drilling performance. The essence of .tungsten carbide button distribution optimization on drills is based on keeping the excellent drilling properties and has the least number of buttons and the best location. Practically, a reasonable distribution of tungsten carbide button is beneficial for reducing the consumption of rock drilling, improving the drilling rate and in case of the wear of cutting buttons early to ensure the best cutting performance. Take DTH drill as an example, the bottom center of the distribution by the number of buttons surrounded, and one of the lowest penetration rates determines the drill bit ROP (rate of penetration), and the lowest life expectancy button also determines the life of the drill bit.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Nonstoichiometric Compound Tungsten Bronze
- Details
- Category: Tungsten Information
- Published on Thursday, 05 May 2016 15:33
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Shielding For Sterilization Of Medical Devices
- Details
- Category: Tungsten Information
- Published on Wednesday, 04 May 2016 18:29
With the development and application of modern science and technology, radiation technology has gradually entered people's daily life and played a vital role in the power generation, industrial, medical and other aspects. Radiation sterilization is a successful example of radiation technology, which is mainly used for the disinfection of disposable medical supplies and medical instruments by using ionizing radiation to kill pathogenic microorganisms (including viruses) to eliminate their poison. The secret of radiation sterilization is the radioactive isotope Co-60. Co-60 is a radioactive isotope of cobalt element that can decay into Ni-60 through β decay while emitting high-energy, high speed electron and two beams of gamma rays. Radiation sterilization is the use of these gamma rays to irradiate bacteria and viruses hiding in the medical supplies and medical equipment, resulting in physiological dysfunction, making it cannot able to grow and develop, or even causing death. The medical instruments to be disinfect by using radiation are mainly blood, surgical instruments, artificial heart and lung, suture materials, injection equipment, transfusion equipment, catheters, bandages, dressings, birth control appliances.
Gamma ray, also called gamma radiation, is extremely high-frequency electromagnetic radiation and therefore consists of high-energy photons. When the body is irradiated with gamma-rays, gamma rays can enter the human body and erode the organic molecules (such as proteins, nucleic acids and enzymes organic molecules which constitute living cells), disturbing the chemical process inside human body, and even leading to cell death. Thus, medical workers should take certain precautions during the process of radiation sterilization of medical devices. In addition to time protection (reduction of exposure time), distance protection (away from the radiation source as far as possible), putting a shielding between man and sources to weaken the intensity of the radiation is also a good protective measures. The radiation shielding generally used for shielding radiation is tungsten alloy radiation shielding.
Due to the radiation sterilization is widely used in disinfection of medical devices in recent years, thus tungsten alloy radiation shielding for sterilization of medical devices can be used to shield radiation produced during the process of radiation sterilization. According to relevant research, the shielding performance of a metal material increases with the increasing of its density, therefore tungsten alloy radiation shielding for sterilization of medical devices has great shielding effectiveness with the high density of tungsten alloy, so that can efficiently absorb and shield radiation produced by the process of sterilization of medical devices, to weaken the intensity of the radiation, and prevent medical personnel from being subjected to radiation damage.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Alcohol and Tungsten Bronze Powder Photochromic Effect
- Details
- Category: Tungsten Information
- Published on Wednesday, 04 May 2016 17:42
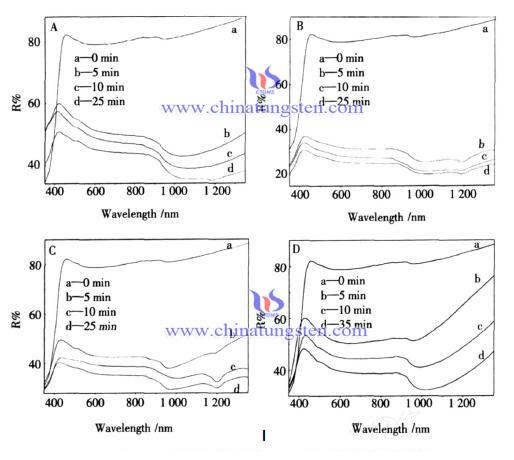
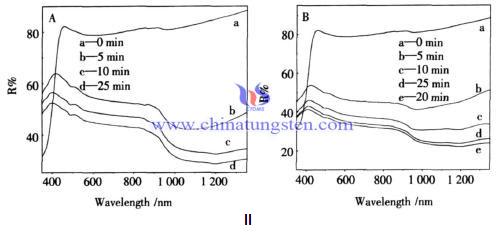
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Contained Low-Temperature Coal Tar Hydrodesulfurization Catalyst
- Details
- Category: Tungsten Information
- Published on Wednesday, 04 May 2016 16:41
 The high price brought by the dwindling oil resources has provided an excellent opportunity for coal chemical industry. Coal tar hydrodesulfurization catalyst is facing several difficulties in this stage:
The high price brought by the dwindling oil resources has provided an excellent opportunity for coal chemical industry. Coal tar hydrodesulfurization catalyst is facing several difficulties in this stage:Firstly, the high oxygen content, the water produced in the deoxygenation process is not conducive to the catalyst activity, stability and strength;
Secondly, high content of gum and residual carbon which results in deactivation of the catalyst and reactor fouled;
Thirdly, high content of sulfur and nitrogen in coal tar; Fourthly, coal tar contains a lot of aromatic hydrocarbons which can’t be removed deeply, and therefore a suitable acidic of catalyst is required.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com