Welding Tungsten Boat
- Details
- Category: Tungsten Information
- Published on Thursday, 12 May 2016 10:43
 Welding tungsten boat is also known as heating wolfram boat. Welding is connected directly to connecting position of the two metals together by the melting at high temperature. Heating and pressing five pieces of tungsten plates at a high temperature so that the connection can be melted together, cooling it after welding, forming the final shape of the boat. Welding of pure wolfram is generally divided into two processes: melting welding and brazing. During the melting welding, molten liquid of two workpieces will mix together under interaction of temperature field and gravity but without any pressure. Until the temperature decreases, the melted portion will condense, two workpieces will be firmly welded together to complete the welding. Brazing use metal material which has a lower melting point than the base material as the brazing filler metal, Heating the weldment and brazing filler metal to make its melting point higher than the melting point of brazing filler metal and lower than the melting temperature of the base material, using liquid brazing filler metal to wet the base metal, filling gap of joint and connect the base material. While welding, the joint of the wolfram plates is called soldering seam. Both sides of it would influenced by heating during soldering and organization and performance would change, this area is known as the heat affected zone.
Welding tungsten boat is also known as heating wolfram boat. Welding is connected directly to connecting position of the two metals together by the melting at high temperature. Heating and pressing five pieces of tungsten plates at a high temperature so that the connection can be melted together, cooling it after welding, forming the final shape of the boat. Welding of pure wolfram is generally divided into two processes: melting welding and brazing. During the melting welding, molten liquid of two workpieces will mix together under interaction of temperature field and gravity but without any pressure. Until the temperature decreases, the melted portion will condense, two workpieces will be firmly welded together to complete the welding. Brazing use metal material which has a lower melting point than the base material as the brazing filler metal, Heating the weldment and brazing filler metal to make its melting point higher than the melting point of brazing filler metal and lower than the melting temperature of the base material, using liquid brazing filler metal to wet the base metal, filling gap of joint and connect the base material. While welding, the joint of the wolfram plates is called soldering seam. Both sides of it would influenced by heating during soldering and organization and performance would change, this area is known as the heat affected zone.
Soldering is a fabrication or sculptural process that joins materials, usually metals or thermoplastics, by causing fusion, which is distinct from lower temperature metal-joining techniques such as brazing and soldering, which do not melt the base metal. In order to improve the quality of soldering wolfram boat, users can use argon, carbon dioxide and other gases to avoid atmosphere so that it can protect the arc and rate of furnace hearth while welding. Finally, a boat shape is formed by welding.
Its size is relatively large, so it can be used for large-scale evaporation device or a large number of material evaporation. Compared to riveting tungsten boat, soldering W-boat can save material of 10% -20%, and it doesn’t need to scribing, drilling, assembling, and other complex processes. Since welding tungsten boat is made of two or more plates, which cannot be made on one time, so the preparation time will be longer.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sintering Tungsten Boat
- Details
- Category: Tungsten Information
- Published on Thursday, 12 May 2016 10:40
 Sintering wolfram boat is to firing anhydrous wolframic acid to produce tungsten powder, which is an important carrier for making it. Or loading ammonium paratungstate (APT) in firing W-boat to make wolfram powder, the APT is white crystal, which include schistose and acicular, it is applied in the manufacturing process of tungsten metal powder by anhydrous wolframic acid or blue tungsten oxide.
Sintering wolfram boat is to firing anhydrous wolframic acid to produce tungsten powder, which is an important carrier for making it. Or loading ammonium paratungstate (APT) in firing W-boat to make wolfram powder, the APT is white crystal, which include schistose and acicular, it is applied in the manufacturing process of tungsten metal powder by anhydrous wolframic acid or blue tungsten oxide.
Sintering tungsten boat is mainly used in the production of tungsten powder. Burning ammonium paratungstate into yellow tungsten oxide or blue tungsten oxide, and then make wolfram powder in hydrogen. During the manufacturing process, tungsten oxide should be placed in a W-boat and then put in the reduction furnace. After recycling the hydrogen, remove the carrier. Fritting is divided into three stages, namely the low temperature burn-in step, the medium temperature firing step and keeping warm in a high temperature. In the low-temperature burn-in step is mainly to volatilize the gas and moisture content which is adsorbed in wolfram boat. Anhydrous wolframic acid is a raw material of wolfram powder.
Process for preparing tungsten powder can be divided into two stages. The first procedure is that the anhydrous wolframic acid is reduced to tungsten dioxide at a temperature of 500 ℃ -700 ℃. The second procedure is that tungsten dioxide is reverted to the wolfram powder under the temperature of 700 ℃ -900 ℃. Purity, particle size, size distribution and other properties of the reduction of W-powder, which primarily rely on the reduction process. When you restore a wolfram powder in a tube furnace, temperature of reduction, charging capacity of tungsten oxide of W-boat, moving speed of W-boat, the flow rate of hydrogen and moisture content of hydrogen content are the main factors to influence the speed of reduction. Particle size of wolfram powder will become thicken as the temperature rises.
While firing, sintering tungsten boat can remove a portion of the harmful impurities, such as sulfur, potassium, sodium and others. This type of W-boat reduces the adverse effects of harmful ingredients (water, oxygen, nitrogen) of the product under vacuum fritting. It is in favor of eliminating the adsorbed gas and the residual gas of the pores which can promote post- fritting shrinkage. However, due to the high temperature, fritting stove would emit large amounts of waste heat.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Shielding Used For Radiation Technology
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 18:08
 With the development and application of nuclear technology, radiation technology shows great potential in the medical, radiation processing, power generation, food security, industrial testing and other aspects. Radiation technology is a subject that is closely linked to the fields of high polymer materials, environmental science, biotechnology and medicine. It main uses radiation (such as gamma rays, X-rays, neutrons, etc.) emitted by radiation source to interact with matter to produce activated atoms and activated molecules which will react with matter both in both in physically and chemically, thereby improving the quality and performance of materials or leading to the degradation, polymerization and crosslinking modification of substances and preparation of new substances. The characteristics of radiation technology include high added value, low energy consumption and wide application, radiation technology is widely used in the modification of semiconductor and polymer, food preservation, and radiation sterilization of medical supplies and the like. Radiation technology can be categorized as radiation crosslinking, radiation curing, radiation vulcanization, radiation degradation, radiation grafted.
With the development and application of nuclear technology, radiation technology shows great potential in the medical, radiation processing, power generation, food security, industrial testing and other aspects. Radiation technology is a subject that is closely linked to the fields of high polymer materials, environmental science, biotechnology and medicine. It main uses radiation (such as gamma rays, X-rays, neutrons, etc.) emitted by radiation source to interact with matter to produce activated atoms and activated molecules which will react with matter both in both in physically and chemically, thereby improving the quality and performance of materials or leading to the degradation, polymerization and crosslinking modification of substances and preparation of new substances. The characteristics of radiation technology include high added value, low energy consumption and wide application, radiation technology is widely used in the modification of semiconductor and polymer, food preservation, and radiation sterilization of medical supplies and the like. Radiation technology can be categorized as radiation crosslinking, radiation curing, radiation vulcanization, radiation degradation, radiation grafted.
Radiation technology has brought radiological hazards while bringing the great facility to people. The largest long-term health risk of radiation is cancer. Radiation can destroy the mechanism in vivo which can prevent cancer, so that greatly increases the risk of cancer. Radiation can also enter the body through a variety of ways, causing internal radiation damage, leading to fatigue, dizziness, insomnia, skin redness, ulceration, bleeding, hair loss, leukemia, vomiting, diarrhea and other symptoms. In addition, radiation will increase aberration rate and hereditary disease incidence, to affect the health of future generations. Exposure to radiation will lead to genetic changes, once the DNA in reproductive cells are damaged, the new generation will inherit the altered gene of mother, thus increasing the risks of defects in the offspring. It is understood that the fetal embryos and fetuses are more sensitive to radiation, exposure to radiation before embryo implantation can increase the late fetal mortality rate; while in the case of exposure to radiation during organogenesis, the increased rates are elevated rate of fetal malformations and neonatal mortality.
Tungsten alloy shielding is widely used to absorb and shield radiation generated by the use of radiation technology. Experts have found that the radiation shielding capability of a material is closely related to its density, higher density means better shielding capability to shield and absorb the radiation. Since the tungsten alloy has a higher density compared with other radiation shielding materials (such as lead), tungsten alloy shielding exhibits better radiation shielding capability. Tungsten alloy shielding has high density also means that under the same shielding ability, tungsten alloy shielding is smaller and thinner.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Hydrodesulfurization Catalyst - Regeneration Flue Gas Treatment 2/2
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 18:05
 3. Preparation of the finished catalyst
3. Preparation of the finished catalyst| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Hydrodesulfurization Catalyst - Regeneration Flue Gas Treatment 1/2
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 18:02

| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
TB Raman Spectra Characterization and Photocatalytic Property
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 17:59
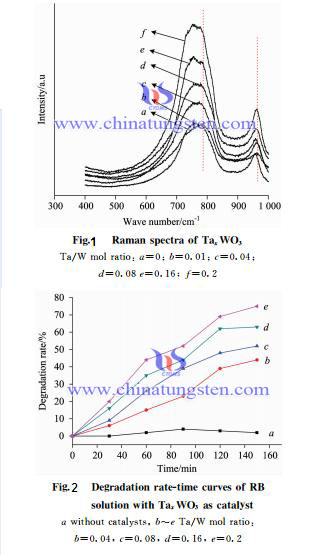
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Anvil Description (2/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 16:57
Synthetic diamond process can be specifically divided into two methods, direct and indirect method. Direct method uses instantaneous dynamic temperature and high pressure, high temperature and static two mixed techniques such as graphite as raw material to C directly into the diamond (meet under static pressure> 13GPa, temperature of 2000K and holding time> 1min; under dynamic conditions, pressure> 20 GPa); the indirect method uses static high temperature and pressure technology to react C elements with the specific metal and obtain synthetic diamond (the transition process is molten metal or alloy + graphite <=> C atom + metal atom or group of atoms <=> diamond + metal or alloy and some carbides).
Domestic synthetic diamond industry mainly is based on hexa-orientation anvil, which belongs to static catalyst method. The basic principle is use six tungsten carbide anvil in six surfaces, while the scope of the high-pressure cavity by the pyrophyllite hexahedron composed of such a graphite cavity under the catalytic effect of high temperature and press into a diamond. Pyrophyllite tetrahedron is a hydrous aluminum silicate layered structure, in the process of synthesis plays role in pressure transmission, sealing and insulation effect; Catalyst acts as a catalyst, can effectively promote the synthesis of diamond, and reduce the pressure required for the synthesis temperature, a common catalyst material Ni70Mn25Co5 alloy.
Anvil diameter is generally φ80mm - φ40mm, density 14.70-14.80g / cm3, hardness of about HRA89.5, diameter of the particles (2-3μm). Sintered tungsten carbide anvil service life is about 5,000 times ten thousand karats consumption> 3kg. Compared with the domestic synthetic diamond industry, foreign-related enterprises have begun to adopt YG12x fine particles carbide diamond anvils, hardness generally reach more than HRA90.5, bending strength of 3000MPa, the average service life is more than 8,000 times, ten thousand karats consumption <1.5kg. Therefore, by grain refinement to improve tungsten carbide anvil density, toughness, flexural strength and prolong the service life of, reduce the consumption, which is a inevitable trend anvil of anvil development.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Anvil Description (1/2)
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 16:55
Diamond is a kind of the hardest mineral consists of pure carbon in nature. It has many advantages, such as higher hardness, excellent wear resistance, which can be widely used in cutting, grinding and drilling industries. Early in the 18th century people began the study of synthetic diamond, but until the last century was able to achieve the preparation of artificial diamond precedent. Tungsten carbide anvil is a key component of the production of synthetic diamond, and it should withstand the alternating loads of high temperature and high pressure, whose life span will directly determines the cost of diamond synthesis production.
So it has higher requirements to tungsten carbide anvil properties: 1. high hardness and compression properties (HRA >85, above 5000MPa); 2. high strength and toughness (tungsten carbide anvil beveled at work entails considerably greater tensile stress> 35kg / mm2); 3. excellent heat resistance, wear resistance and corrosion resistance (in the process of synthetic diamond, the transition temperature of C element is about 2000℃, and the added catalyst is not only beneficial for the synthesis, but also not react with the anvil).
Tungsten carbide grades suitable for anvil include YG6, YG8, YG12x and so on. YG6 tungsten carbide is composed of 94% WC + 6% Co, mainly based on WC powder and Co powder with medium grains. Higher hard phase WC content to ensure the compression performance of tungsten carbide anvil. But the lower content of the binder phase Co will affect the tensile strength of the anvil, which will be easily cracked in the process of synthesis diamond. Furthermore, tungsten carbide YG6 anvil has some difficulties in the content of C controlling, which prone to appear free C or η phase and affect the properties of the anvil; YG8 tungsten carbide with medium grain consist of 92% WC and 8% Co, which is not only has excellent compression performance, but also has excellent tensile strength and longer service life, so fewer cracks occur in the early stage; YG12x tungsten carbide is a kind of alloy with fine grains. Under certain circumstances of Co content, the finer granularity of WC, correspondingly the density, the hardness, the compression performance and other composite properties will be remarkably improved. Therefore, refining the grain is a kind of ideal process of tungsten carbide anvil.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
LECO S144DR Carbon Sulfur Analyzer and Tungsten Granule
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 11:09
Tungsten granule is considered as flux of LECO S144DR carbon sulfur analyzer which plays an important role in enhancing liquid flow, controlling the temperature of combustion oxidation while analyzing carbon and sulfur. Generally, its shape is fine stuff with a size of 0.4-0.8μm. Tungsten granule accelerates the combustion reaction of inorganic material which has the function in light the fire, combustion-supporting. It can also melt of the oxidizing material of the sample’s surface to make flux have a good fluidity, which is conducive to oxidize the carbon and sulfur of the sample in a short time. Compared to CS230, the greatest different is that S144DR is a tube furnace which belongs to external heating, its sample is burned in a high-temperature tube furnace. CS230 is a high-frequency heating furnace, high-frequency furnace using a metal sample to generate eddy current in a high frequency producing CO2 and SO2 in oxygen.
LECO S144DR carbon sulfur analyzer mainly uses a size 0.7-1.2μm of magnesium perchlorate as a desiccant, and glass wool is used for filtering dust. Purity of oxygen should reach above 99.5% whose outlet pressure is 0.26MPa. Its basic structure consists of a tube furnace, infrared absorption system, purification system, constant-temperature system, gas system, steady flow systems, and data processing and control system. Melting and combustion of the sample is mainly carried out in a tube furnace. Infrared absorption system is used for measuring SO2, the purification system is used for purifying carrier gas and the reaction gas to remove its dust, impurities and moisture. Thermostat system allows temperature of measured gas and the detector cell keep constant. The reaction gas is offered by the gas system. Steady flow system is mainly to control the flow of the carrier gas, the main data processing and control system is to control of the instrument and calculate sulfur content.
Operational principle of this type of analyzer is first to weighing a certain quality of the sample into the flow of oxygen, combusting in a high-temperature furnace, sulfur is converted to sulfur dioxide, flowing with oxygen through the infrared absorption cell, and detect the sulfur.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Operational Principle of LECO CS230 Carbon Sulfur Analyzer and Tungsten Granule
- Details
- Category: Tungsten Information
- Published on Wednesday, 11 May 2016 11:06
For the determination of carbon, the main way is to combust carbon which can be converted to carbon monoxide and / or carbon dioxide in oxygen. Using infrared absorption spectroscopy of carbon dioxide and carbon monoxide in oxygen to determine carbon. The determination of sulfur is first to burn sulfur in oxygen which is converted to sulfur dioxide. Then, sulfur is detected infrared absorption spectroscopy of sulfur dioxide. The electronic control part of the carbon sulfur analyzer consists of infrared, air flow control panel, and stimulus airflow control panel, and power control rack, incubator heating control panel, computers and other electronic components.
Path of the carrier gas of the analysis of the gas flow is to let oxygen pass the rare earth oxide copper oxide of inlet to remove the carbon monoxide methane and other impurities of carrier gas. To remove its carbon dioxide and moisture with soda asbestos and magnesium perchlorate. Then, it would pass the oxygen lance and carrier gas inlet into the furnace end. Gas of samples which is generated in combustion tube which is made of fused silica would go with carrier gas after magnesium perchlorate to remove its moisture, and then, entering into detector cell of sulphur. After that, transfer sulfur dioxide into sulfur anhydride and remove it. Detection of carbon dioxide of carrier gas would be in process of high carbon and low carbon at the both ends of gas path of the instrument.
When LECO CS230 carbon sulfur analyzer works with tungsten particles, its operational principle is first to put a certain quality of sample into a high-frequency magnetic field which has been passed through an oxygen stream. The sample and flux would be induced and heating, combusting in oxygen, carbon and sulfur of sample reacts with oxygen to produce carbon dioxide and sulfur dioxide which will accompany with the carrier gas into the gas system. They will first to reach the sulfur dioxide detector testing pool to determine sulfur, and then using thermal oxidation of copper to convert carbon monoxide into carbon dioxide. Next, sulfur dioxide will be converted into sulfur trioxide, and then it would be absorbed by the plastic which has a strong adsorbability. Then, the sample gas would go through the high and low content of carbon dioxide of the infrared detector cell to detect carbon.

| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com