Tungsten Alloy Radiation Shielding For Radioactive Decay
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 19:14
 Radioactive decay, also known as nuclear decay or radioactivity, is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha particles, beta particles, gamma rays and conversion electrons. A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element.
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which the nucleus of an unstable atom loses energy by emitting radiation, including alpha particles, beta particles, gamma rays and conversion electrons. A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element.
No matter be which kind of decay, radioactive rays will be generated. Exposure of large dose or exposure of a certain dose for a long-term both will cause harm to human body. Such as γ-rays can penetrate human skin and get into human body, to interact with cells in vivo, thus eroding complex organic molecules (such as proteins, nucleic acids, enzymes). These molecules once destroyed, the normal chemical processes in the human body will get disturbed, and cell death will be increased in severe cases. Other radiation also can penetrate the human skin to cause radiation damage. When radiation enters the body, it will destroy the molecular structure of the genetic material DNA, leading to congenital malformations or leukemia, and it will also damage the reproductive system, central nervous system and endocrine system and cause cataracts, cancer, and a number of radiation sickness.
Tungsten alloy radiation shielding can be used to shield the radioactive rays produced by the process of radioactive decay. Tungsten alloy radiation shielding for radioactive decay is made up of tungsten heavy alloy with high density. Since the shielding property of a material is closely related to its density. The higher the density is, the better the shielding performance is. Therefore Tungsten alloy radiation shielding for radioactive decay has excellent shielding property, so that can absorb and shield the radioactive rays produced by the process of radioactive decay in a high degree, protecting humans from radiation damage. The density of lead is lower than tungsten alloy, so comparing to the same weight of lead shielding, tungsten alloy radiation shielding is smaller and thinner. In addition, tungsten alloy radiation shielding for radioactive decay is non-toxic and environmentally friendly shielding material.
| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Prepares Non-Supported Desulfurization Catalyst - Urea Melting Reaction Method
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 17:17
 Urea melting reaction method is mixing the precursor metal components and urea, reacted in a molten state urea to remove excess urea to obtain the catalyst particle with nano-pores and high specific surface area. The example that using urea melting reaction method and taking tungsten trioxide as raw material to prepare non-supported desulfurization catalyst is as following:
Urea melting reaction method is mixing the precursor metal components and urea, reacted in a molten state urea to remove excess urea to obtain the catalyst particle with nano-pores and high specific surface area. The example that using urea melting reaction method and taking tungsten trioxide as raw material to prepare non-supported desulfurization catalyst is as following:| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Non-Supported Hydrodesulfurization Catalyst Material--WO3
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 17:15
 With the declining oil quality and industrial emissions standards becoming critical, the issue to develop ultra-clean fuel is increasingly difficult, and very necessary. Vary the reaction conditions (such as raising the temperature and pressure of the reaction, reducing airspeed, etc.) can achieve the goal of deep hydrodesulfurization process, however, they will result in the loading cost increased, product quality and the amount of processing oil reduced, also the other issues, therefore, the development of new deep hydrodesulfurization catalyst is imperative.
With the declining oil quality and industrial emissions standards becoming critical, the issue to develop ultra-clean fuel is increasingly difficult, and very necessary. Vary the reaction conditions (such as raising the temperature and pressure of the reaction, reducing airspeed, etc.) can achieve the goal of deep hydrodesulfurization process, however, they will result in the loading cost increased, product quality and the amount of processing oil reduced, also the other issues, therefore, the development of new deep hydrodesulfurization catalyst is imperative.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Rotary Burrs Description (2/2)
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 16:52
Compared with developed country, there are several problems in tungsten carbide rotary burrs of our country: 1. The defect of materials (includes the purity of raw material, the machining parameters control, etc.,); 2. The most used tungsten carbide rotary burr is not integral, which uses tungsten carbide as head, bearing steel as shank and fabricated rotary burr by welding. So it will have problems in welding firmness; 3. The precision and operability of CNC machining equipment; 4. The angle, shape and tolerances of the edge and insufficient concentricity.
In order to further improve the cutting properties and extend the service life of tungsten carbide rotary burr, we analyze it from the mechanism of cutting and forming. Generally, Carbide rotary burrs edge back is composed of straight or curved: the straight edge is easy to be processing, but the strength of the edge is lower; the intensity and difficulty of processing polyline edge back has a certain increase; although curve edge has a difficulty in processing, it can ensure that the strength at any point of the cross-section is substantially equal. Therefore, we need to find out a kind of edge that based on the strength and also easy to be fabricated.
Besides, the spacing between two edges also plays a important role in the process. If the spacing is too large, the corresponding cutting and machining efficiency decreases; if the spacing is too small, it can not ensure the scrap removing, which will have an effect on edge blocking and the efficiency of tungsten carbide rotary burr. The overall milling process can be divided into three steps: the initial wear stage, normal wear stage and rapid wear stage. On each of the different stages through reasonable cutter angle (rake angle, relief angle, the main angle, relief angle and blade angle) choice, you can reduce the wear of the cutting force and cutting heat brought in the same under the cutting conditions, cutting into full play, enhance overall efficiency and precision. Overall, improvements and material from the rotary file rotary file blade teeth should have high hardness, high strength, high wear resistance, good impact resistance and high temperature conditions can still ensure good cutting performance, but also to facilitate the processing of the material; The tolerances on the edge to be precise enough to meet the requirements of surface roughness.
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Rotary Burrs Description (1/2)
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 16:50
Tungsten carbide rotary burrs consist of the hard phase WC and the binder phase Co, which is also known as tungsten carbide assorted cutter or high-speed molding cutter and so on. It is mainly cooperated with high-speed electric grinder and pneumatic tools. Due to its processing hardness can reach more than HRA 85, it can be widely applicable to the most of materials, such as common cast iron, steel, copper, aluminum, marble, alloy steel, carbon steel and other metal or non-metal materials. The principle is that tungsten carbide rotary burr clamped on high-speed rotary tool, which achieves the cutting performance by the pressure and feed rate controlling.
Since tungsten carbide rotary burr has excellent cutting properties and long life span, it has a broad application prospect, such as finishing for metal mold cavity; castings, forgings, welding components the existence of flash, burrs, weld cleaning; A variety of mechanical parts chamfering, machining grooves and keyways; pipeline, flow channel, the inner surface of the hole parts cleaning and grinding; the sculpture of A variety of metal or non-metallic materials, etc.
According to the shape of head, tungsten carbide rotary burr can be divided into cylinder (A), cylindrical-shaped (C), round arch (F), spherical (D), round-shaped torch (H), 90° conical (K), 60° conical (J), 38° conical (M), arc-shaped disk (T), inverted conical (N), tapered round (L), tapered tip (M), drum-shaped (B), ellipse (E), curved arrow-shaped (G), tapered flat-shaped (S), half arc-shaped (W) and so on. And the common grades used in tungsten carbide rotary burrs are YG6, YG8 and YG10x, which has excellent impact toughness, wear resistance and is suitable for cold heading, cold punch, cold mold fabricating.
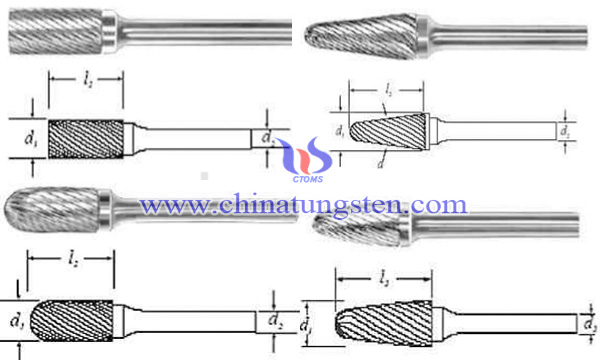
| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Filament Poisoning of IG Tungsten Filament in Freeman Ion Source in Working
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 16:40
 Freeman ion source has been 30 years of history, it is still one of the most widely scope of application and the most commonly used ion source. Because it can make most of the elements generate a very small focus and Ma level ion beam, and it has a wide energy range so that this kind of ion source becomes a common choice. On semiconductor ion implantation applications, the source has a good performance and a simple operation and other excellent characteristics, which is widely used as a doped of silicon devices. Freeman ion source has been successfully applied in many aspects of ion implantation apparatus for decades, including the production of a milliampere level of a variety of metal ion beam. Throughout the ion implantation apparatus for industrial using, approximately 95% are using it.
Freeman ion source has been 30 years of history, it is still one of the most widely scope of application and the most commonly used ion source. Because it can make most of the elements generate a very small focus and Ma level ion beam, and it has a wide energy range so that this kind of ion source becomes a common choice. On semiconductor ion implantation applications, the source has a good performance and a simple operation and other excellent characteristics, which is widely used as a doped of silicon devices. Freeman ion source has been successfully applied in many aspects of ion implantation apparatus for decades, including the production of a milliampere level of a variety of metal ion beam. Throughout the ion implantation apparatus for industrial using, approximately 95% are using it.
While working, it is possible to have the phenomenon of poisoning. On the basis of a chemical treatment to remove the surface oxide layer and the chemical cleaning, load a diameter of 1.5mm industrial tungsten wire in ion source injection machine. Adopting the 99.9% purity of MoCl5 as working substance which is easy to absorb moisture, on the other hand, it is easy to be broken down. During the test, it is found that IG tungsten filament would poison in Working. Whiling work, the insulation of insulator is breakdown, causing the short-circuited of electrodes between the filament and the arc chamber, resulting in the filament poisoning. During the MoCl5 injection process, if the amount of intake air is too high, the ionization rate of the arc chamber would be reduced, a part of MoCl5 will be deposited on the directly heated cathode, causing poisoning phenomenon. Because the temperature of electron-tube heater which is near the both ends of the arc chamber wall is relatively low, it is easier to deposit MoCl5 at its ends. Therefore, poisoning of this process often occurs at both ends, it can be found through the thicker filament. But the poisoning of the middle part of the filament is not so obvious so it also has the ability to emit electrons. Most reasons for filament poisoning are the higher vapor pressure under the low temperatures. In addition, molybdenum and tungsten are two completely miscible metals, it is conducive to the growth of the film at the initial stage, but the amount of intake air also makes a great impact of filamentary cathode.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Filament Poisoning of IG Tungsten Filament in Freeman Ion Source in Degassing
- Details
- Category: Tungsten Information
- Published on Monday, 09 May 2016 16:36
 Freeman ion source is a kind of ion source which is drew forth by electron collision. Its filament and the narrow slit of ion extraction are parallel and located directly behind ion source. The length of the filament is corresponding to length of narrow slit. Under the interaction on magnetic field of filament and the external magnetic field, forcing the electrons generated by the heat of the cathode do the screw motion in the non electric field plasma. About 100 Oe variable magnetic field can be generated by a magnetic field. The breakdown of ion source is that due to some problems which would cause the ion source is not working properly. In particular, when the metal ions are implanted, the working substance is mostly metal chlorides, easily deposited on the ion source insulator, causing a short circuit between the electrodes. In addition, filament poisoning, intake duct blockage and corrosion damage is common problems of ion sources.
Freeman ion source is a kind of ion source which is drew forth by electron collision. Its filament and the narrow slit of ion extraction are parallel and located directly behind ion source. The length of the filament is corresponding to length of narrow slit. Under the interaction on magnetic field of filament and the external magnetic field, forcing the electrons generated by the heat of the cathode do the screw motion in the non electric field plasma. About 100 Oe variable magnetic field can be generated by a magnetic field. The breakdown of ion source is that due to some problems which would cause the ion source is not working properly. In particular, when the metal ions are implanted, the working substance is mostly metal chlorides, easily deposited on the ion source insulator, causing a short circuit between the electrodes. In addition, filament poisoning, intake duct blockage and corrosion damage is common problems of ion sources.
While using an ion source injection machine for modification process of material surface, the ion source may not work properly so that it needs to be shut down for inspection and maintenance. If there exists operation materials that deposited on the filament during inspection which will lead to a partial or entire filament become thicken slowly. The filament resistance will be weakened, reducing its ability to emit electrons which would cause arc blowout that is also known as arc extinction. When the voltage exceeds a certain value, it would lead to an arc discharge. For example, when the breaker is disconnected under the circumstances of electricity, however, due to the high voltage on the fracture, it will generate result in arc extinction, and the circuit continues to conduct which is very dangerous. This phenomenon is referred to as filament poisoning.
After a chemical treatment and the chemical cleaning, tungsten filament is put in ion source. Adopt MoCl5 whose purity is 99.9% as the working substance to analyze poisoning of an IG tungsten filament. In the process of degassing, heating the filaments slowly, and then start to vaporization, through the heat conduction of intake-tube to make the part of the work material be gasified. After entering the arc chamber, most of MoCl5 gas will be extracted in beam induced exit of arc chamber. The remaining part is deposited on the tungsten which would decompose into Mo and Cl2 quickly at a high temperature. Cl2 will be pulled out, but Mo is still deposited on the filament, leading poisoning. During the inspection, find that filament become thicker evenly and has a serious poisoning.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ceriated Tungsten Electrode Manufacturing
- Details
- Category: Tungsten Information
- Published on Friday, 06 May 2016 19:20
 Manufacturing ceriated tungsten electrode usually uses powder metallurgy method. And the production processes are as follow, mixing-ball-milling-reduction-pressing-sintering-swaging-drawing-straightening-cutting-grinding. In order to improve ceriated electrode properties, the production process should be strictly control and some production parameters should be adjusted. There we will introduce how to manufacturing ceriated electrode, which is little different form original manufacturing processes.
Manufacturing ceriated tungsten electrode usually uses powder metallurgy method. And the production processes are as follow, mixing-ball-milling-reduction-pressing-sintering-swaging-drawing-straightening-cutting-grinding. In order to improve ceriated electrode properties, the production process should be strictly control and some production parameters should be adjusted. There we will introduce how to manufacturing ceriated electrode, which is little different form original manufacturing processes.
First, the doped quantity is different, the cerium oxide from 1.0% up to 4.5% by weight by powder metallurgy, besides, characterized in that a cerium salt solution is added to tungsten trioxide. After pressing the product obtained is then dried and calcinated at 300 to 800°C, reduction with two-steps hydrogenous reduction method in a hydrogen tubular furnace, whereby the first step is carried out at 750°C and the second step at 900°C. Using two-steps hydrogenous reduction method can obtain the maximum grain size of the tungsten powder which is less than 8µm. Then the obtained tungsten cerium powder is pressed into bar form by isostatic pressing machine. Pressing by isostatic pressing machine can make product more densification and can improve electrodes strength. After pressing presintered the bar in molybdenum furnace with hydrogen passing through it, again the bar is sintered at high temperature electrically and under hydrogen protection. When we got the blank bar is not finish yet, we should operate a series subsequent process to make tungsten bar become thinner and shorter which is convenient for customers using. On the other hand, after subsequent deformation process, electrode’ mechanical properties will better. So the bar is hot rotary forged, and annealed, when necessary, during rotary forging, furtheron drawn under Ø3.00 mm diameter to wire of necessary diameter under the gas heated furnace, and annealed, when necessary, during the drawing.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
WO3 Hydrodesulfurization Catalyst Prepares Low-Sulfur Gasoline
- Details
- Category: Tungsten Information
- Published on Friday, 06 May 2016 18:10
 The sulfur and thiol contents in cracking (FCC) gasoline are respectively 200~1600 ug/g, 30~200 ug/g. FCC gasoline is a major component of some refineries, its mixing ratios can even up to 80% ~ 90%. Therefore, the key to meet the new clean gasoline specifications is to reduce the sulfur content of the FCC gasoline and mercaptans. Hydrodesulfurization catalyst containing tungsten trioxide can be used to handle the full range FCC gasoline to produce low-sulfur gasoline. In which, the gasoline feedstock does not required to fractionate, also the intermediate separation process does not required after RSDS, with the simple process and easy to operate.
The sulfur and thiol contents in cracking (FCC) gasoline are respectively 200~1600 ug/g, 30~200 ug/g. FCC gasoline is a major component of some refineries, its mixing ratios can even up to 80% ~ 90%. Therefore, the key to meet the new clean gasoline specifications is to reduce the sulfur content of the FCC gasoline and mercaptans. Hydrodesulfurization catalyst containing tungsten trioxide can be used to handle the full range FCC gasoline to produce low-sulfur gasoline. In which, the gasoline feedstock does not required to fractionate, also the intermediate separation process does not required after RSDS, with the simple process and easy to operate.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Additive Affects WO3 Hydrodesulfurization Catalyst Property
- Details
- Category: Tungsten Information
- Published on Friday, 06 May 2016 18:08
 To meet the increasingly stringent sulfur and nitrogen content requirements, developing the hydrodesulfurization (HDS), hydrodenitrogenation (HDN) catalyst with excellent properties is an effective way. The active components of hydrodesulfurization catalysts are generally the transition metal of tungsten, molybdenum, cobalt and their oxides (e.g., tungsten trioxide). Supported and non-supported are the mainly two types of tungsten trioxide hydrodesulfurization catalysts; in addition, their performance affected by additives to some extent.
To meet the increasingly stringent sulfur and nitrogen content requirements, developing the hydrodesulfurization (HDS), hydrodenitrogenation (HDN) catalyst with excellent properties is an effective way. The active components of hydrodesulfurization catalysts are generally the transition metal of tungsten, molybdenum, cobalt and their oxides (e.g., tungsten trioxide). Supported and non-supported are the mainly two types of tungsten trioxide hydrodesulfurization catalysts; in addition, their performance affected by additives to some extent.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com