Tungsten Powder Made from Ammonium Paratungstate -One-step Reduction Method 2/3
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 21:20

| APT Supplier: Chinatungsten Online ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Powder Made from Ammonium Paratungstate -One-step Reduction Method 1/3
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 21:14
 1. Basic introduction
1. Basic introduction| APT Supplier: Chinatungsten Online ammonium-metatungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Calcium Hydroxide Causticizing-Precipitating Scheelite from Sodium Tungstate Solution
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 21:11

| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Removing Silicon, Phosphorous and Arsenic from Sodium Tungstate Solution
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 21:05
| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sodium Tungstate Catalyzes Aryl Nitriles to Prepare Aryl Amides
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 21:00

| Sodium Tungstate Supplier: Chinatungsten sodium-tungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Hydrogen Reduction Tungsten Trioxide Crafts
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 19:55
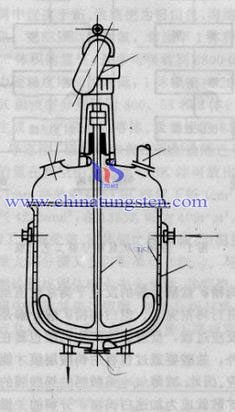
Early preparation method is based on the classic tungsten powder and hydrogen as a reducing agent to restore the tungsten trioxide powder into metal tungsten powder, and this method is easy to control high purity tungsten powder and tungsten powder particle size, etc. In the past, it has been widely adopted in the world. Now, it is replaced by hydrogen reduction of tungsten blue.
Tungsten oxide reduction reactions are endothermic reaction, it is favorable for the restore response when temperature rises. Low tungsten oxide has a higher stability than the more expensive tungsten oxide; therefore, we use the two-stage reduction in the industry, and the second reduction temperature must be higher than the first temperature reduction. Tungsten and oxygen lines, there are tungsten trioxide WO3, tungsten oxide WO 2.9, tungsten oxide WO 2.72, tungsten oxide WO 2 and so on tungsten oxide. The total reaction of tungsten trioxide tungsten powder preparation is generally carried out step by step, that WO3 (yellow), WO 2.90 (blue), WO 2.72 (purple), WO2 (brown), W (gray).
Hydrogen reduction of tungsten trioxide, also known as yellow tungsten process, the most popular method is the continuous in the industrial production. Generally, we use the two-stage reduction system to take middle or partial fine particles and more uniform particle size tungsten powder, which is prepared by the WO3, W is prepared by the second WO2. At the time of preparation of coarse tungsten metal powder, it is used for some reduction, W is prepared by WO3.
 Fine Tungsten Powder Principles
Fine Tungsten Powder Principles
(1) A lower reduction temperature;
(2) A more stable temperature gradient;
(3) Push the boat slower speed;
(4) A small amount loaded boat or thinner material layer;
(5) Greater hydrogen flow and smaller hydrogen humidity;
(6) WO3 particles finer materials.
Coarse Tungsten Powder Principles
(1) The reduction of high temperature;
(2) A large temperature gradient;
(3) Faster push the boat speed;
(4) Large amount loaded boat or thick charge layer;
(5) The smaller and larger hydrogen flow humidity.
Hydrogen reduction of tungsten trioxide production of tungsten carbide powder has primarily for tungsten powder and tungsten material for processing of tungsten powder, tungsten powder of these two methods are different.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Alloy Balance Wheel Screw CounterweightsⅠ
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 17:56

| Tungsten Alloy Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Gold-plated Products Daily Maintenance
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 17:53

| Tungsten Gold Plated Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Gold-plated Products And Gold Products Identification
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 17:50

| Tungsten Gold Plated Supplier: Chinatungsten Online www.tungsten-alloy.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Effect of Reversal Time on Tungsten Copper Powder Granularity
- Details
- Category: Tungsten Information
- Published on Monday, 04 January 2016 17:24
Analyze the effect of tungsten copper powder by changing operation cycle of high-energy planetary ball milling reversal time. Generally, the operation time of planetary ball mill machine is 1-999min, and the positive and negative reversal cycle is 1-99min. In order to achieve the best ball milling effect, the rotating speed, ball milling time, the ball (size, collection, etc), the size and amount of sample and accessories choosing are all important.
Observe tungsten copper composite powder by scanning electron microscope (SEM) in different reversal time, we can find that tungsten copper composite powder particles of irregular shape, indicating that as reducing the time change, the powder grain size smaller, and appears reunion phenomenon. The shorter reversal time, the agglomeration phenomenon of tungsten copper composite powder is more obvious. Tungsten-copper composite powder and other rolling, cold rolling and upsetting effect shaft structure, ball and ball, between the ball and the tank wall under, along with reducing the time change, tungsten, copper W-Cu powder in plastic deformation, a sheet-like composite powder is formed under cold welding. Furthermore, since the strain hardening and microscopic increase plastic composite powder decreased, resulting in its fracture refinement, the particles after fracture and constantly repeated cold welding and fracture occurs again, tungsten, copper particles are more uniformly mixed composite powder becomes even finer particles.
In the process of solid solution, a small amount of copper (Cu) diffuse into tungsten (W) phase and form solution, which enlarges the distance of crystal face. However, with the decreasing reversal time, residual stress is increasing and the distance of crystal face is decreasing. It indicates that the impact of residual stress plays a leading role in the process. This is due to the high energy ball milling process, the tungsten-copper composite powder in ball rolling, cold rolling and shearing strong process, resulting in a severe plastic deformation has great stress and strain. Within the grains formed a lot of dislocation, distortion and other microscopic defects, and increase the microscopic strain, dislocation of a large number of tangles, and to further promote the formation of sub-cellular structures and lead to reduced grain size. Meanwhile, due to a large amount of crystal defects inside the particles, the atomic activity and energy storage systems increases, which promote the formation of solid solution.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com