Cerium Tungsten Electrodes and Thorium Tungsten Electrode Comparison
- Details
- Category: Tungsten Information
- Published on Monday, 22 August 2016 15:41

With the continuous improvement of welding, cutting, spraying techniques, people require new performance requirement on electrode material such as better active substances and content to reduce the work function to improve the arc performance; higher chemical stability to use in more welding atmosphere and so on.
Material Properties Comparison:
Compared with thorium tungsten electrode, the work function and α-ray dose of cerium tungsten electrode are lower than thorium tungsten electrode, which has better performance advantages and no radiation. Under the same cutting conditions, when the nozzle is leakage, thorium tungsten electrode wear has significantly increased, but little change in cerium tungsten electrodes. This phenomenon also happen in gas discharge lamp, when the oxygen content increase, cerium tungsten electrode has slight change, which can be seen it has good antioxidant properties.
Arc performance comparison:
It was determined that thorium tungsten cathode pressure is small than cerium tungsten, 2% Tn-W13.0V, 2% Ce-W12.0V. The service life is related with cathode spot. In xenon stroke light compared these two electrodes found, cerium tungsten end face has slight cathode spot and has smaller loss, and the service life is longer than thorium tungsten. In emission current density, cerium tungsten arc column euphotic belt is bright and long and emission current density is higher than thorium tungsten. When the diameter is large, the minimum arc stability of various electrodes is similar, with the reduction of diameter, the cerium tungsten electrode arc performance is good than other electrodes. The lowest arc voltage of cerium tungsten is 12V, thorium tungsten arc voltage is 30V, so cerium tungsten arcing is more simple.
From the above comparison found cerium tungsten electrode has better arc performance. This is mainly because it has low work function, so the cathode drop is low and cathode spot is small and the emission is concentration. Meanwhile, when the arc current increases, the arc through large current will produce large magnetic field centripetal force and the arc is compressed, so that the emission current density of cerium tungsten electrodes is increase.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Mesoporous WO3-CeO2-ZrO2 Solid Superacid Catalyst
- Details
- Category: Tungsten Information
- Published on Monday, 22 August 2016 11:29
 Mesoporous WO3-CeO2-ZrO2 solid superacid catalyst is based on zirconium oxide as the main component, by introducing a rare earth element Ce to maintain a stable tetragonal phase of ZrO2; WO3 as an accelerator to replace sulfate and avoid the loss of sulfate to cause deactivating; at the same time, the introducing of mesoporous structure will get high mesoporous material surface area and pore volume to have more active center, stronger adsorption and mass transfer, thus to get access to mesoporous with high catalytic activity composite solid superacid. Solid superacid refers to the solid acid with its strength greater than 100% of concentrated sulfuric acid, that is, its Hammett acidity function H0≤-11.93. Solid superacid has strong acid, good activity, high selectivity, easy to separate from the product, does not corrode equipment, little environmental pollution, is a green and efficient, safety and environmental protection of the new catalyst.
Mesoporous WO3-CeO2-ZrO2 solid superacid catalyst is based on zirconium oxide as the main component, by introducing a rare earth element Ce to maintain a stable tetragonal phase of ZrO2; WO3 as an accelerator to replace sulfate and avoid the loss of sulfate to cause deactivating; at the same time, the introducing of mesoporous structure will get high mesoporous material surface area and pore volume to have more active center, stronger adsorption and mass transfer, thus to get access to mesoporous with high catalytic activity composite solid superacid. Solid superacid refers to the solid acid with its strength greater than 100% of concentrated sulfuric acid, that is, its Hammett acidity function H0≤-11.93. Solid superacid has strong acid, good activity, high selectivity, easy to separate from the product, does not corrode equipment, little environmental pollution, is a green and efficient, safety and environmental protection of the new catalyst.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Defect State Nano Structure Tungsten Oxide Catalyst Is Invented
- Details
- Category: Tungsten Information
- Published on Friday, 12 August 2016 18:01
USTC(University of Science and Technology of China) recently announced that professor Xiong Yujie research group designed a kind of tungsten oxide nano structure in defect state based on inorganic solid accurate preparation of chemical and use crystalline defect project. Under broad spectrum illumination, it shows fine oxidation coupling catalytic property which is expected to realize low cost and low-energy organic chemical technology. Tungsten oxide has good photocatalytic property due to its special structure, it has already been used as varies of catalyst and also other industrial fields.
Most of catalytic reaction is based on the application of precious metal oxide and motivated by burning of petroleum and coal. It has disadvantages of high cost and high energy consuming. Compared to precious metal catalyst, metal oxide has benefits of low cost. However, it shows shortcomings in oxygen molecular system which can not capture solar energy and pass it into oxygen molecular.
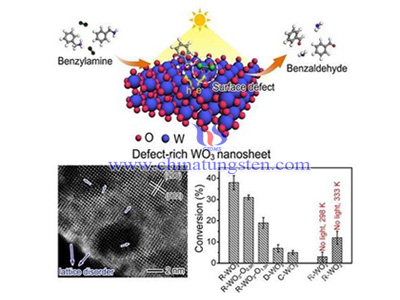
In order to solve the problem, Xiong Yujie research group designed a kind of nano structure tungsten oxide. Usually metal atom of metal oxides has coordination saturation property, it can not active oxygen molecular by chemical absorption. During the research, construct of oxygen vacancy defect overcomes the shortcomings and accelerate photo electron transact from metal oxides catalyst to oxygen molecular. Defect state also broadens light absorption range of photocatalyst which enables it to capture solar energy in visible light and near infrared area. The two big steps realize the valid capture of solar energy and energy transaction, solves the bottleneck problems of oxides catalyst in the organic synthesis of photocatalytic activity.
Based on this acknowledgement, researchers are able to adjust solar energy to drive organic oxygen coupling reaction based on crystalline defect project. It provides possibility to use solar energy take place of heat source organic synthesis, and make an improvement for design of photocatalyst material.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Electrode Manufacturing
- Details
- Category: Tungsten Information
- Published on Wednesday, 10 August 2016 14:22

At present, the fuel cells usually use Pt / C catalyst or Pt alloy catalyst as the oxygen reduction catalyst, but Pt and its alloy is expensive and lack of resources, which greatly limits its application range. Tungsten carbide electrode is a non-noble metal catalyst and the catalytic property is similar with platinum and has CO poisoning resistance, so it as catalyst used in electrochemistry field is more and more widely.
Tungsten carbide electrode manufacturing:
1. Weighed a certain amount of ammonium paratungstate (APT) to make 10% aqueous solution, placed aqueous solution into spray drying apparatus at room temperature for spray-drying microspheres treatment can obtain ammonium paratungstate powder.
2. Placed ammonium metatungstate powder in quartz boat of tube type resistance furnace leads to mixed gas of H2 and CO (H2 as reducing gas and CO as carbon source). And then the furnace temperature was raised to 400 ℃, heat preservation for 1~2 hours, then heated to 900 ℃heat preservation for 6 ~7 hours.
3. After reaction finished, leads to N2 with naturally cooling to obtain tungsten carbide (WC).
4.Catalyst layer production: Uniform mixed WC, activated carbon, polytetrafluoroethylene emulsion (60%) by 10: 1: 3 proportion, added appropriate absolute ethyl alcohol for 5 minutes ultrasonic dispersion and then placed in 80℃ water to heat with stirring makes the mixture to reunite. Placed the aggregate on two-roll mill repeatedly rolling to film, its thickness is 0.2mm.
5. Waterproof layer preparation: uniform mixed acetylene black, anhydrous sodium sulfate and PTFE by 1: 1: 1 mass ratio, and then follow the steps of catalyst layer production process to obtain waterproof layer, its thickness is approximately 0.2mm.
6. To superpose breathable waterproof layer, catalyst layer and current collector layer by layer and pressed on hydraulic machine 10MPa to obtain tungsten carbide gas diffusion electrode.
| Tungsten Metals Supplier: Chinatungsten Online www.tungsten.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Factors of Hydrothermal Method Preparing Hexagonal Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 10 August 2016 09:33
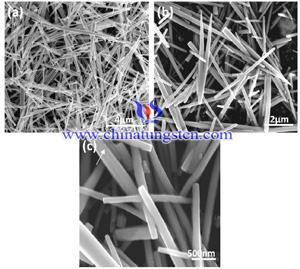 Hydrothermal process is carried in a special closed reaction vessel (reactor), the aqueous solution as medium, by heating the reaction vessel to create a high-temperature, high-pressure reaction environment to make the generally insoluble or poorly soluble material to dissolve and recrystallization, and then by filtering, washing, drying and other separation means to get the ultra-fine, high-purity particles. Hydrothermal method for preparing hexagonal tungsten trioxide, its crystalline form is affected by many factors, such as pH value, hydrothermal temperature and additives and others, the following will specifically analyze the impact of various factors.
Hydrothermal process is carried in a special closed reaction vessel (reactor), the aqueous solution as medium, by heating the reaction vessel to create a high-temperature, high-pressure reaction environment to make the generally insoluble or poorly soluble material to dissolve and recrystallization, and then by filtering, washing, drying and other separation means to get the ultra-fine, high-purity particles. Hydrothermal method for preparing hexagonal tungsten trioxide, its crystalline form is affected by many factors, such as pH value, hydrothermal temperature and additives and others, the following will specifically analyze the impact of various factors.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Hexagonal Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Friday, 05 August 2016 09:58
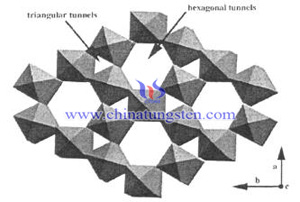 Tungsten trioxide is a unique n-type semiconductor material, that makes it one of the few oxide semiconductors which are easy-to-realize quantum size effect, and performances the excellent properties in photocatalysis, electrochromic, photochromic, gasochromic and other aspects, thus widely used in fields of chemical sensor, fuel cell, light catalyst and so on. Tungsten trioxide has orthogonal, monoclinic, cubic, hexagonal and other crystal structure. Among them, hexagonal tungsten oxide causes many concern because of its special hexagonal passage; many metal ions can be embedded in this hexagonal channel, thereby forming a hexagonal tungsten bronze which exhibits potential application in anode material and rechargeable lithium-ion battery.
Tungsten trioxide is a unique n-type semiconductor material, that makes it one of the few oxide semiconductors which are easy-to-realize quantum size effect, and performances the excellent properties in photocatalysis, electrochromic, photochromic, gasochromic and other aspects, thus widely used in fields of chemical sensor, fuel cell, light catalyst and so on. Tungsten trioxide has orthogonal, monoclinic, cubic, hexagonal and other crystal structure. Among them, hexagonal tungsten oxide causes many concern because of its special hexagonal passage; many metal ions can be embedded in this hexagonal channel, thereby forming a hexagonal tungsten bronze which exhibits potential application in anode material and rechargeable lithium-ion battery.| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Nozzle Used for Oil Drilling
- Details
- Category: Tungsten Information
- Published on Wednesday, 03 August 2016 16:47
With the development of the oil industry and increasing energy demands, the oil drilling related technology has been developing constantly. In the early years, oil drilling mainly uses impact drilling by human, mechanical drilling and rotary drilling, etc., and at a selected location down or side surface drilled to a diameter of the cylindrical bore underground reservoirs. These types of high-pressure pneumatic drill, DTH drill and cone drill can be used with tungsten carbide buttons to improve the drilling efficiency and extend the service life of the drill. Now the researchers through studying deeply and find a kind of more effective technology that high-pressure abrasive water jet technology for the deep and edge reservoirs exploiting. High pressure abrasive water jet takes high-pressure water as the carrier, adds a certain amount of abrasive particles to greatly enhance the impacting force and the rock formation cutting efficiency also be remarkably improved. So it has a broader application prospect.
Tungsten carbide nozzle plays an important role in high pressure abrasive water jet. It has high hardness, high strength and excellent wear and corrosion resistance. Generally, oil drilling process is always under a high confining pressure environment, so the nozzle needs to withstand the high-speed impact of high pressure grinding compound and it will be wore and failure easily. Such this situation, common materials like steel nozzle will be prone to thermal deformation or be cracked, which needs to change the nozzles frequently and the drilling efficiency decreased. Especially it is extremely inconvenient that remove and replace the drill bit in deep drilling. In addition, to mount tungsten carbide material in the parts most likely to be worn is also a kind of method to improve the properties and extend the service life of nozzle. Furthermore, in the manufacturing process of tungsten carbide nozzle, it can improve the hardness and wear and corrosion resistance further by decreasing the granularity of WC grains based on no reducing the material toughness.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Carbide Nozzle Used for Sand-blasting
- Details
- Category: Tungsten Information
- Published on Wednesday, 03 August 2016 16:20
Sand-blasting treatment is also called shot peening treatment, is a kind of workpiece surface treatment process that powered by compressed air, the beam through the high-speed jet spray material (such as the common copper sand, quartz sand, silicon carbide, iron sand, sea sand, etc.) to the high-speed jet processing workpiece surface. During the process, the surface or the shape of the workpiece has been changed by the cutting and impacting action and obtain certain roughness and cleanliness. Furthermore, the sand-blasting process also improves the mechanical properties and fatigue resistance, which also enhances the binding force and durability of the coating layer and is beneficial for the leveling and decoration of the coatings.
At present, sand-blasting process widely used in mechanical rust removing and polishing. And the sand-blasting equipment used includes inhaled dry blasting machine, pressure-blasting machine dry or liquid blasting machine. Regardless of what type of blasting equipment, tungsten carbide nozzles are the one of the essential components. Compared with other materials, such steel nozzle, tungsten carbide nozzle has higher hardness, higher strength and better performance in wear and corrosion resistance. But there are two problems in the sand-blasting process. One is difficulty in sand suction caused by the poor vacuum degree of inlet wet sand and the small inlet diameter; the other is within the blowhole water mixed with sand from the entrance to the straight pipe does not match or sand mixed with water nozzle wear inappropriate material selection problems caused by straight pipe. So the main criteria used to judge carbide blasting nozzle includes material selection, the length of the diameter of the sand suction tube diameter, the diameter of the blowhole sand-water mixture from the straight pipe sections and other factors. In addition, in the sandblasting process, pay attention to controlling the distance well nozzle and the workpiece, and the position of the welding time control to eliminate the residual stress of the welding surface. And corrosion inhibitors can be added to a mixture of water blasting process to prevent the secondary oxidation of metal surface.

| Tungsten Carbide Supplier: Chinatungsten Online tungsten-carbide.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Trioxide Nanowire CO Gas Sensor Preparation
- Details
- Category: Tungsten Information
- Published on Wednesday, 03 August 2016 11:26
 CO, the product of incomplete combustion of carbon fuels and cracking reactions occur at high temperatures, oxidation reaction ect. has become one of the major sources of air pollution, which greatly threats human life and health and environmental protection. The body of CO maximum allowable limit is 10-4, while in Europe the provisions of the environment CO must not exceed 10-5. So, the detection and controlling of CO is becoming urgent, tungsten trioxide based gas sensor is considered to be the most promising sensor for detecting NOx, O2 and NH3 and other poison gases, due to its simple structure, low cost, high sensitivity and gas sensing. This paper presents a method for preparing tungsten trioxide nanowire CO gas sensor which shows as follow:
CO, the product of incomplete combustion of carbon fuels and cracking reactions occur at high temperatures, oxidation reaction ect. has become one of the major sources of air pollution, which greatly threats human life and health and environmental protection. The body of CO maximum allowable limit is 10-4, while in Europe the provisions of the environment CO must not exceed 10-5. So, the detection and controlling of CO is becoming urgent, tungsten trioxide based gas sensor is considered to be the most promising sensor for detecting NOx, O2 and NH3 and other poison gases, due to its simple structure, low cost, high sensitivity and gas sensing. This paper presents a method for preparing tungsten trioxide nanowire CO gas sensor which shows as follow:| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Two-Dimensional Tungsten Disulfide/Tungsten Trioxide Monohydrate Lateral Heterojunction Preparation
- Details
- Category: Tungsten Information
- Published on Monday, 01 August 2016 18:54
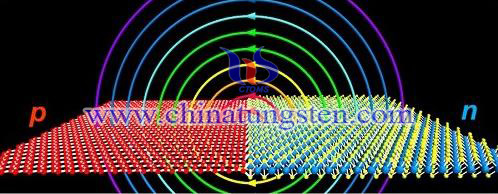
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com