Extraction of Tungsten from Acidic High-Phosphorus Solution for Producing APT
- Details
- Category: Tungsten Information
- Published on Saturday, 03 July 2021 18:29

Tungsten is one of the most important high-tech metals, and its high-purity products are vital for developing advanced materials. Wolframite and scheelite have been used as main resources for tungsten extraction. However, the high-quality wolframite and scheelite resources reduced with the continuously exploitation and utilization and the tungsten deposits with high content of impurities must be exploited. As one of the main impurities, phosphorus significantly affects the quality of the ammonium paratungstate (APT) products. In order to obtain qualified APT, the phosphorus impurity contained in tungsten deposits must be removed in the process of tungsten hydrometallurgy.
Potassium-Doped Tungsten with High Thermal Shock Resistance Prepared from APT
- Details
- Category: Tungsten Information
- Published on Tuesday, 29 June 2021 01:27
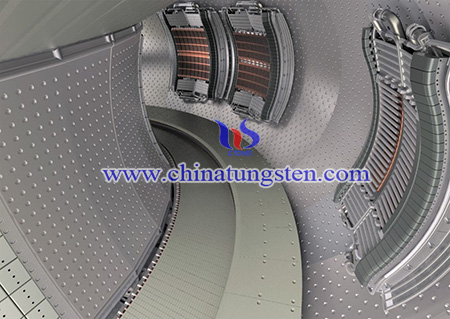
As one of the most promising candidates for plasma-facing material (PFM) in future fusion reactors, tungsten was extensively investigated in recent years. However, even though tungsten has many advantages like high melting point, high thermal conductivity, low tritium inventory and low erosion rate under plasma loading, there still exist some disadvantages such as high ductile–brittle transition temperature (DBTT), low recrystallization temperature (RCT), irradiation induced hardening, and so on.
Ammonium Paratungstate Applied in Photocatalytic Degradation of Herbicides
- Details
- Category: Tungsten Information
- Published on Monday, 28 June 2021 22:06
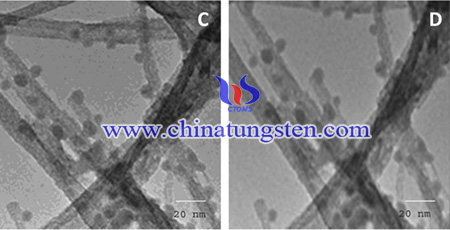
Many types of herbicides and pesticides can be used for the growth inhibition of weeds and protection of crops from insect pests. However, through the transfer of wastewater that contains residual herbicides and pesticides, groundwater and rivers can be polluted. Many crops contain 2,4-dichlorophenoxyacetic acid (2,4-D), as it was considered a main ingredient for more than 1500 herbicides and pesticides. It is a carcinogenic and highly toxic pollutant that causes injury to the heart and central nervous system, and because of its high biological and chemical stability, it is very difficult to decompose
Quasi-Spherical Nanosized Tungsten Prepared by APT
- Details
- Category: Tungsten Information
- Published on Sunday, 27 June 2021 21:27

Tungsten has been widely used as structural materials in aerospace, military, and energy industries owing to its brilliant characteristics such as high mechanical strength and melting point, well thermal conductivity, and good resistance to oxidation. Generally, bulk tungsten is fabricated by powder metallurgy process and high density and fine grain size are critical to achieve outstanding mechanical properties such as the hardness of sintered compacts. Due to its high melting point of 3420 °C, the powder metallurgy process usually requires very high sintering temperatures of 2700–2800 °C to get near fully dense tungsten, which always leads to over growth of the grain size and deteriorates the mechanical properties.
Preparation of Carbon-Coated Tungsten Trioxide with Enhanced Photocatalytic Activity Using Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Sunday, 27 June 2021 17:32

With the rising global energy supply and related environmental issues brough by burning of fossil fuels, various materials including nitrides, oxides, metals, metal chalcogenides and phosphides have been developed. Tungsten oxides (WOx) caught a lot interest because of their earth-abundance, highly tunable composition, high chemical stability at an appropriate pH value, and excellent electrical conductivity. Individually, WOx semiconductors has excellent performance in photocatalytic degradation of many organic pollutants.
Unfortunately, it has the drawbacks of low light adsorption and relatively narrow band gap. An effective and practical strategy is to design tungsten oxide composites with carbon. improve the photocatalytic ability by preparing the amorphous carbon-coated tungsten trioxide (WO3) with a high defect concentration. In order to overcome these disadvantages, WO3 had been doped with carbon to enhance its photocatalytic activity.

The fabrication method of carbon-coated tungsten trioxide is conducted as following procedures: All raw materials were of analytical grade and commercially available and used as received without further purification. The commercial WO3 was used for comparison.
0.01 mol ammonium paratungstate (APT), 0.24 mol ammonium nitrate (NH4NO3, ⩾ 99.0%), 0.1 mol glycine (C2H5O2N) and various contents of glucose (C6H12O6) were dissolved into deionized water, and then the solution was continuously stirred for a homogeneous state. The mixture was placed on an electrical furnace in air for thermal processing. The solution was evaporated with the formation of a gelatinous mass during heating. Upon further heating, the resultant mass swelled suddenly, accompanied by the release of a lot of gases. The whole process of swelling and combustion of gel indicates the non-explosive and self-propagating exothermic reaction, which took several minutes. The samples prepared with 0.017, 0.033, 0.05 and 0.067 mol glucose were described as G1, G2, G3 and G4, respectively.

In summary, amorphous carbon-coated tungsten oxide nanocrystals were synthesized via with enhanced photocatalytic activity is prepared using ammonium paratungstate as starting material. Carbon-coated tungsten oxide existed in the form of round particles with size of ~ 150 nm and the WO3-x nanorods with the length of ~ 20 nm and diameter of 5 nm. The synthesized carbon coated WO3 showed a considerable rate for organic degradation under UV–visible light as well as good stability. This excellent performance is attributed to the synergistic effect of the amorphous carbon and the large amounts of defects. Moreover, the material demonstrated good photocatalytic stability under visible light after 5 recycling degradation of MB.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com
Synthesis Process of Eu3+:Kla(WO4)2 Red Phosphors Using APT
- Details
- Category: Tungsten Information
- Published on Sunday, 27 June 2021 11:29
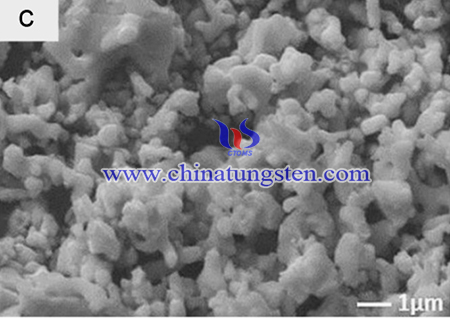
Lanthanide-doped luminescent materials have been studied extensively because of their wide applications in optoelectronic devices due to their interesting optical properties originating from the electron transitions among the 4f shell. Among these, alkali rare earth tungstates constitute a large family of inorganic compounds with the general formula ARE(MO4)2. These compounds possess tetragonal and monoclinic symmetries. Their structural diversity provides these crystals with numerous physical and chemical properties.
Fabrication of Nanosized Ammonium Paratungstate by Solid-gas Phase Reaction
- Details
- Category: Tungsten Information
- Published on Saturday, 26 June 2021 22:38
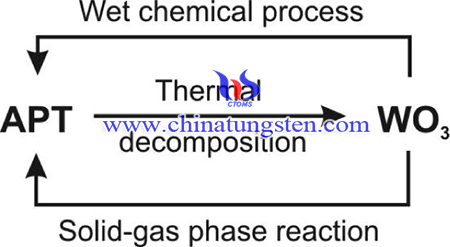
The ammonium paratungstate (APT) is the most important starting material in the tungsten industry, where usually its most stable APT·4H2O form is used. From APT tungsten oxides, tungsten carbides or tungsten metal can be prepared. Among others, tungsten oxides are widespread catalysts, photocatalysts, and gas sensors.
APT Utilized in Regeneration of V2O5-WO3/TiO2 SCR Catalyst
- Details
- Category: Tungsten Information
- Published on Saturday, 26 June 2021 01:56
Nitrogen oxde poullution has been caught a lot attention due to its negative influence on environment. fly-ash emitted from coal combustion flue gas always carries various toxic substances, such as SO2, alkali metals and alkaline-earth metals. They tend to lower the efficiency of NOx removal and shorten the operating life-time of the SCR catalyst. V2O5-WO3/TiO2 has been greatly used in NOx removal applications.
WO3 Catalyst Being Prepared by Ammonium Paratungstate for DSSCs Applications
- Details
- Category: Tungsten Information
- Published on Saturday, 26 June 2021 01:48

Dye-sensitized solar cells (DSSCs), as a renewable energy source, have attracted considerable attention due to their unparalleled merits including low cost, choice of color, light weight, packing technology, environmental compatibility, and acceptable photoelectric conversion efficiency (PCE).
Synthesis of V2O5-WO3/Tio2 Catalysts from Ammonium Paratungstate in SCR Applications
- Details
- Category: Tungsten Information
- Published on Saturday, 26 June 2021 00:54

In urea-SCR of passenger Diesel vehicles, the NOx reduction step of urea-SCR is usually catalyzed by redox zeolites. Technology for stationary sources r emains to be dominated by catalysts that contain V2O5 and WO3 supported on TiO2 (anatase). They are meanwhile also applied for mobile sources, in heavy utility vehicles, where they are adapted to the different targets. Thus, V2O5-WO3/TiO2 catalysts had been synthesized from ammonium paratungstate (APT) for SCR applications.


 sales@chinatungsten.com
sales@chinatungsten.com