Extraction of Tungsten from Acidic High-Phosphorus Solution for Producing APT
- Details
- Category: Tungsten Information
- Published on Saturday, 03 July 2021 18:29
Tungsten is one of the most important high-tech metals, and its high-purity products are vital for developing advanced materials. Wolframite and scheelite have been used as main resources for tungsten extraction. However, the high-quality wolframite and scheelite resources reduced with the continuously exploitation and utilization and the tungsten deposits with high content of impurities must be exploited. As one of the main impurities, phosphorus significantly affects the quality of the ammonium paratungstate (APT) products. In order to obtain qualified APT, the phosphorus impurity contained in tungsten deposits must be removed in the process of tungsten hydrometallurgy.
Extraction of tungsten from acidic high-phosphorus solution has been conducted for APT production, the ammonium tungstate solution meeting the requirements of producing high-quality APT. The synthesis method of tungsten extraction from acidic high-phosphorus solution is as following steps:
Aliquat 336 (methyl trioctyl ammonium chloride) and 2-Octanol were purchased from Aladdin Reagent Co., Ltd. A certain amount of 2-Octanol was used as co-solvent. Therefore, the organic phases were composed of different volume contents of Aliquat 336, 2-Octanol and kerosene. The sodium tungstate (Na2WO4) was purchased from Aladdin Reagent Co., Ltd. and then diluted into the mixture solution of sulfuric acid (H2SO4) and phosphoric acid (H3PO4) to prepare aqueous phase. The content of phosphorus and tungsten contained in aqueous phase are 300 g/L and 10 g/L, respectively. Ammonium bicarbonate solution (NH4HCO3) was used as stripping agents. The magnesium chloride was used as precipitant to remove phosphorus from strip liquor. All the reagents are of analytical grade and all the solutions with specified concentrations were prepared using deionized water.

The solvent extraction experiments were performed by shaking the required volume of organic and aqueous phase in a separatory funnel at room temperature (25 °C). The H2SO4 and NaOH solution were used to adjust pH values of the solution during extraction. The equilibrium pH of the aqueous raffinates was measured after the phase disengagement.
Experimental conditions, including equilibrium pH, extraction time, volume ratio of the organic and aqueous phase (O:A), and volume content of extractant, were investigated to determine the optimal extraction conditions. The extraction efficiency can be calculated according to Eq.
Where E represents the metal extraction efficiency, C1 and C2 the substance concentration in the aqueous phase before and after extraction, V1 and V2 the volumes of aqueous phase before and after extraction.
The extracted organic phase was first washed by deionized water to remove the remaining water phase, and then stripped by ammonium bicarbonate solution. The stripping conditions, such as ammonium bicarbonate concentration, stripping time, volume ratio of the organic and aqueous phase (O:A), temperature and stripping stages, effecting the stripping rate of tungsten has been investigated. The calculation method of stripping rate is similar to that of extraction efficiency.
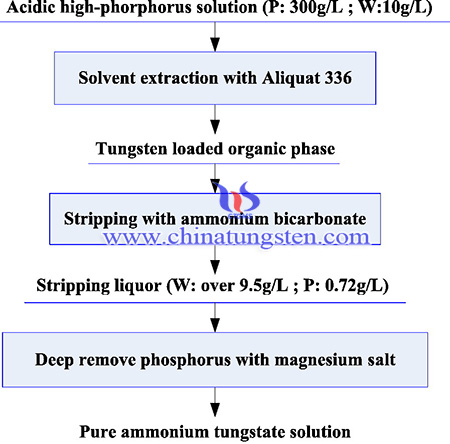
The phosphorus contained in strip liquor was removed deeply by magnesium salt precipitation. A certain amount of magnesium chloride was added into a 500 mL, three-necked and round-bottomed flask containing about 200 mL strip liquor. The flask was placed in a water bath with a stirrer (Chao Yue, DF-101S) and was coupled with a condenser. A desired pH value of the strip liquor was obtained by adding HCl or NH3·H2O solution. The precipitation process was conducted at an agitation speed of 300 rpm under the conditions of changing pH values, temperature and magnesium chloride dosage.
In summary, Extraction of tungsten from acidic high-phosphorus solution has been conducted for APT production, the ammonium tungstate solution meeting the requirements of producing high-quality APT. 99.8% tungsten contained in acidic high-phosphorus solution was extracted with Aliquat 336 in kerosene by one stage. Compared to traditional phosphorus removal—ammonium paratungstate crystallization route, the proposed process in this paper can extract tungsten directly in acidic medium and decrease the quantity of phosphorus through extraction-stripping process, significantly reducing the consumption of alkali and magnesium salt and improving the separation efficiency of tungsten from acidic high-phosphorus solution.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com