rGO/WO3 Composites with Excellent Electrochemical Performance from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Monday, 05 July 2021 03:14
The global energy demand has increased due to rapid population growth. The depletion of energy sources and increase in energy demand have urged researchers worldwide to study the electrochemical energy storages system, such as batteries, conventional dielectric capacitors, and fuel cells. Supercapacitors are one of the electrochemical capacitors that have attracted much research interest because of their long cycle life, high power density, excellent reversibility, environment friendly and higher safety.
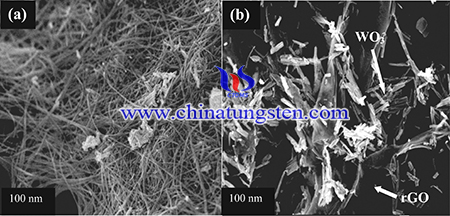
Reduced graphene oxide (rGO) has been reported as a promising material because of its distinctive mechanical, optical, electronic and electrochemical properties. Thus, rGO has been widely used in solar cells, corrosion protection, optoelectronic devices, and bioelectric sensory devices for early detection of leukemia and DNA. In order to find a substitute source for current fossil fuels, rGO/WO3 composites with excellent electrochemical performance have been prepared from ammonium paratungstate (APT).
The synthesis method of rGO/WO3 composites is as below: Graphite powder was pretreated before oxidation to prevent incompletely oxidized GO as a final product. In a typical procedure, 30 mL of concentrated sulfuric acid was poured into a round bottom flask followed by 6 g of K2S2O8, 6 g of P2O5 and 4 g of graphite powder. The mixture was heated at 80 °C in an oil bath under mechanical stirring for 6 h. The mixture was allowed to cool to room temperature, before being diluted with 2 L of deionized water and kept overnight. The precipitate was filtered and dried in a vacuum desiccator overnight.
GO was synthesized using graphite powder as the precursor by modified Hummers' method. The resulting GO was reduced to rGO via a chemical reduction route using hydrazine as the reducing agent. Briefly, 1 g of graphite oxide was dissolved in 500 mL of deionized water and then sonicated for 30 min to form a supernatant GO. The solution was adjusted to pH 10 using 5 M KOH. Approximately, 0.25 mL of hydrazine was added into the above solution and refluxed at 95 °C for 24 h. The mixture was filtered, washed with deionized water and dried in an oven for 24 h to obtain rGO.
In the synthesis of WO3, 1.056 g of APT and 0.935 g of NaCl were dissolved in 30 mL of deionized water under mechanical stirring for 20 min. The mixture was adjusted to pH 2 using 3 M HCl and stirred for another 3 h. About 50 mL of the resulting solution was transferred into a Teflon-lined autoclave and incubated for 24 h at 180 °C. The sediment was filtered and washed with deionized water several times. The precipitate was then dried in an oven at 60 °C for 24 h.
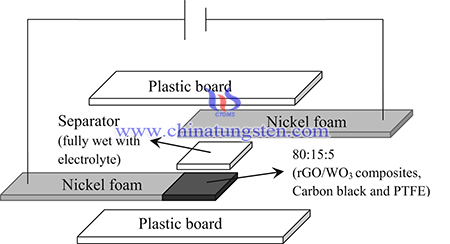
rGO/WO3 composites were fabricated via an electrostatic assembly method. Firstly, 90 wt% rGO was dissolved in 20 mL of deionized water under sonicating for 1 h. Then, 10 wt% WO3 was added into the rGO solution. The mixture was stirred for 24 h and then washed several times with deionized water. The precipitate was filtered and oven dried at 60 °C overnight. The weight ratio of rGO and WO3 were 85:15, 90:10, 95:5 and 97:3 and denoted as rGO/WO3−15, rGO/WO3−10, rGO/WO3−5 and rGO/WO3−3, respectively.
In summary, rGO/WO3 composites with excellent electrochemical performance had been prepared from ammonium paratungstate. A simple process had been utilized to prevent the agglomeration of rGO and improving the electrochemical performance of the electrode in a supercapacitor. The rGO/WO3 nanocomposite demonstrated high specific capacitance of 85.7 F g−1 at a current density of 0.7 A g−1, which increased the specific capacitance by 10% compared with WO3. Based on the results, rGO/WO3−10 exhibited the highest specific capacitance, excellent cycle stability and the lowest values of Rs and Rp, revealing that rGO improved the conductivity of WO3 and resulted in better ion diffusion between WO3 and rGO. This improvement will lead to the extensive use of composite materials in energy storage device applications.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com