Synthesis Process of Eu3+:Kla(WO4)2 Red Phosphors Using APT
- Details
- Category: Tungsten Information
- Published on Sunday, 27 June 2021 11:29
Lanthanide-doped luminescent materials have been studied extensively because of their wide applications in optoelectronic devices due to their interesting optical properties originating from the electron transitions among the 4f shell. Among these, alkali rare earth tungstates constitute a large family of inorganic compounds with the general formula ARE(MO4)2. These compounds possess tetragonal and monoclinic symmetries. Their structural diversity provides these crystals with numerous physical and chemical properties.
Factors such as crystalline size, grain size distribution and morphology have an effect on the luminescent behaviour. sol–gel is a simple, cost effective and versatile process for the preparation of multi-component crystalline metal oxides. Thus, Eu3+:KLa(WO4)2 red phosphors are prepared using ammonium paratungstate (APT) as tungsten source, the product has strong red emission at about 615 nm under the excitation of near UV and blue LEDs regions.
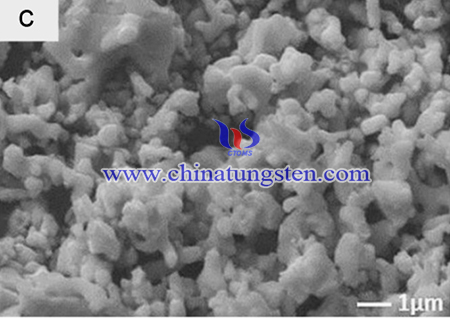
The synthesis process of is as below: Eu:KLW phosphors were synthesized by the Pechini sol–gel method. The reagents KNO3 (99% Merck, India), La(NO3)3 (99.9% CDH, India), Eu(NO3)3 (99.99% Alfa Aesar) and ammonium paratungstate (CDH, India) were used as starting precursors. Citric acid and ethylene glycol were used as chelator and binder respectively.
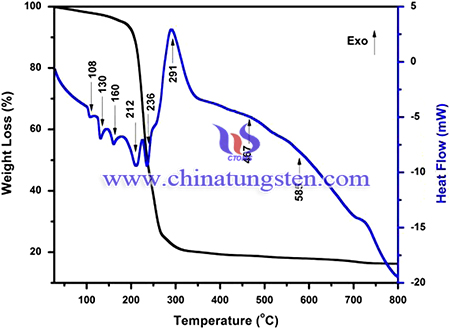
Stoichiometric amounts of metal nitrates (i.e.), KNO3, La(NO3)3, Eu(NO3)3 and ammonium paratungstate were dissolved separately in deionized water. Then, citric acid was added individually with each of the solution of metallic nitrates and tungstate. Degree of chelation of the process was controlled by the molar ratio of citric acid to the metal and this ratio should be greater than one to get uniform distribution of metal ions in the solution and taken as 1:0.9 for the present work. Carboxylic groups of the citric acid chelates the metal ions and results in homogeneous distribution in the solution by the formation of complex ring shaped product around the metal cations [21]. These metal citrates were mixed together and stirred vigorously with constant heating of 80 °C for 20 min. The total pH of the mixed solution was adjusted to 3.5 by adding ammonia solution. The appropriate amount of binder was added to promote the citrate polymerization. Rigid polyester net was formed, which prevents secondary phase segregation of metals during the polymer decomposition process. This mixture solution was dried under constant stirring and temperature to obtain the polymer gel. Then the gel was pre-firied at 250 °C and the obtained powder was further calcined at different temperatures from 500 to 800 °C. The resultant powders were characterized.
In conclusion, Eu:KLW red phosphors were synthesized through Pechini type sol-gel method. Intense red emission corresponding to 5D0→7F2 transition (615 nm) of Eu3+ ions was observed under near-UV (395 nm) excitation. The optimum concentration of Eu3+ with the KLW host for strong red emission was found to be about x=0.40 and the critical transfer distance (Rc) of Eu3+ ions was determined to be 9.50 Å. The lifetime τ of the phosphors was obtained to be 1.52 ms. The CIE chromaticity coordinates (x=0.650, y=0.348) of Eu:KLW (x=0.40) under λex=395 nm was closer to the NTSC values. These results indicate the potential application of these phosphors as red source in WLED׳s.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com