Fabrication of Nanosized Ammonium Paratungstate by Solid-gas Phase Reaction
- Details
- Category: Tungsten Information
- Published on Saturday, 26 June 2021 22:38
The ammonium paratungstate (APT) is the most important starting material in the tungsten industry, where usually its most stable APT·4H2O form is used. From APT tungsten oxides, tungsten carbides or tungsten metal can be prepared. Among others, tungsten oxides are widespread catalysts, photocatalysts, and gas sensors.
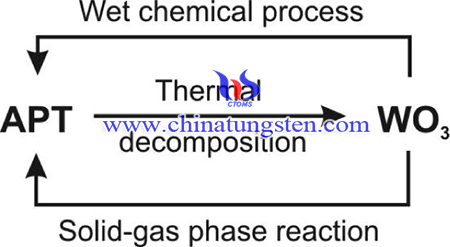
Conventionally, APT is produced by the wet chemical process, which is a multi-step method. The first step is the alkaline digestion, when the tungsten content is extracted from the tungsten ore concentrates, scrap metal or oxides. However, it has several drawbacks. For example, it is very sensitive to the conditions, especially to the pH. moreover, the process has a large chemical and energy requirement.
Solid–gas phase reaction is used to prepare nanosized ammonium paratungstate (40–300 nm), the method has simple process and is feasible to be used in industry. Based on our results the properties of the as-obtained APT are identical to the commercial APT materials, also this is the only method to prepare nanosize APT.
The fabrication method is described as following procedures: The WO3 powders prepared from hexagonal ammonium tungsten bronze (HATB, (NH4)0.33−xWO3−y) were used as precursors in the solid–gas phase reactions. HATB was obtained by heating APT at 400 °C in H2 for 6 h. The precursor tungsten oxides were prepared by thermal decomposition from HATB by controlling the annealing temperature and atmosphere.
The reactions between the precursor tungsten oxide powders and the NH3 and H2O vapors were carried out at room temperature in a sealed plastic box. WO3 powder and aqueous ammonia solution were placed separately into the box, where the reagents were able to react only via the gas phase. The equilibrium partial pressure of NH3 and H2O vapors values were calculated from the concentrations of the aqueous solutions. Using precursors 1–4 the effects of the composition and crystal structure of the tungsten oxides were investigated. From precursors 5–11 the effects of the particle size of the powders and the partial pressure of the ammonia were studied. In each reaction 500 mg WO3 powder and 25 ml aqueous ammonia solution were used. The yield of the reactions was calculated from the mass difference before and after the reaction.
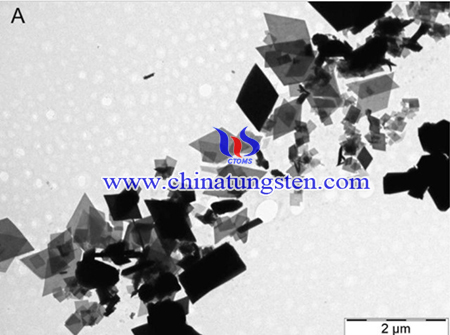
In summary, Solid–gas phase reaction is used to prepare nanosized ammonium paratungstate (40–300 nm), the method has simple process not sensitive to the reaction conditions, in contrast to the more complex and sensitive wet chemical process. These measurements proved that the as-prepared APT is equal to the commercial one. SEM and TEM images revealed that nanosize (40–300 nm) APT was produced for the first time.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com