Ammonium Paratungstate Applied in Photocatalytic Degradation of Herbicides
- Details
- Category: Tungsten Information
- Published on Monday, 28 June 2021 22:06
Many types of herbicides and pesticides can be used for the growth inhibition of weeds and protection of crops from insect pests. However, through the transfer of wastewater that contains residual herbicides and pesticides, groundwater and rivers can be polluted. Many crops contain 2,4-dichlorophenoxyacetic acid (2,4-D), as it was considered a main ingredient for more than 1500 herbicides and pesticides. It is a carcinogenic and highly toxic pollutant that causes injury to the heart and central nervous system, and because of its high biological and chemical stability, it is very difficult to decompose
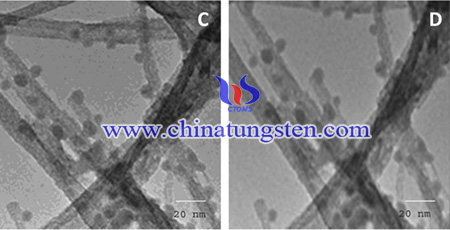
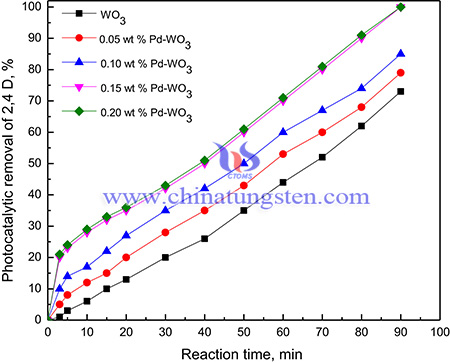
Photocatalytic methods have also been used for the removal of herbicides. Titanium dioxide has been well accepted as a photocatalyst due to its wide band gap and high electron-hole recombination rate, however, cause it to have low photodegradation efficiency. Thus, tungsten trioxide (WO3), which has a narrow band gap, has received a lot attention. Recently, Ammonium paratungstate has used in Pd-doped WO3 nanorods for photocatalytic degradation of herbicides.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com