Formation of Heterotypic Substitutional Solid Solutions (NH4)10–xKx[H2W12O42] · n H2O in the Ammonium Paratungstate ‘Z'/Potassium Paratungstate ‘Z' System
- Details
- Category: Tungsten Information
- Published on Tuesday, 08 December 2015 10:31
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Ammonium Paratungstate Tetrahydrate and Highly Pure Ammonium Paratungstate Tetrahydrate Method Producing
- Details
- Category: Tungsten Information
- Published on Tuesday, 08 December 2015 10:22
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Co-doped Effects on Tungsten Copper Electrode III
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 16:37
Sintering additives, which can dissolve tungsten, can be used for decreasing the porosity of tungsten copper electrode. The most common method is to add active elements, which takes advantage of active elements to dissolve tungsten and improve phase precipitation, round and accumulation, to obtain a high density. It can remarkably improve the properties of tungsten copper, decrease the sintering temperature and shorten the sintering time. The effect of different content of Co element on the hardness of tungsten copper electrode as follow:
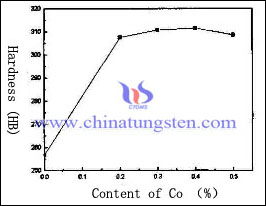
We can learn from the graph that the effect of Co element on the hardness of tungsten copper electrode is similar to the density of tungsten copper electrode, which remarkably increases at the beginning and tends to stabilization or decrease. When the content of cobalt is about 0.3%, the density and hardness of tungsten copper electrode reaches the maximum 16.69g/cm3, and HB311.6.
More infomations about Co-doped effects on tungsten copper electrode, click here:
http://news.chinatungsten.com/en/tungsten-information/81018-ti-10447
http://news.chinatungsten.com/en/tungsten-information/81083-ti-10458
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Co-doped Effects on Tungsten Copper Electrode II
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 16:30
Adding activate element to promote tungsten dissolution, and of the solid phase sintering and liquid phase diffusion densification during sintering tungsten particles dissolved in the liquid phase precipitation, round and accumulation, to obtain a high density. Its addition can remarkably improve the material properties, reduce the sintering temperature and shortening the sintering time.
However, the addition of these elements will be activated in a certain extent, reduce the thermal conductivity and electrical conductivity of tungsten copper electrode. Therefore, this kind of method is suitable for the occasions, which has lower demands of thermal conductivity and electrical conductivity. The effect of different content of Co on the density of tungsten copper electrode as follow:
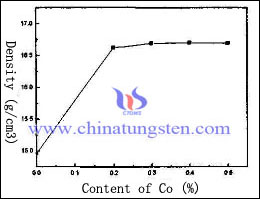
The graph shows that the reaction of tungsten copper electrode density is sensitive to the adding Co element, which increases at the beginning, 14.9g/cm3 rapidly increases to 16.6g/cm3. Afterwards, the content of Co increases constantly, the density of tungsten copper electrode changes a little and has a decreasing trend.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Thermal Decomposition of Ammonium Paratungstate Hydrate in Air and Inert Gas Atmospheres
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 09:50
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Preparation of Coarse and Spherical Tungsten Powders by Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Monday, 07 December 2015 09:47
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Direct Solid State Synthesis of W–Al2O3 Nanostructured Composite Using Ammonium Paratungstate (APT) and Al Powder Mixture
- Details
- Category: Tungsten Information
- Published on Friday, 04 December 2015 14:18
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Growth and Morphology of W18O49 Crystals Produced by Microwave Decomposition of Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Friday, 04 December 2015 14:12
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Co-doped Effects on Tungsten Copper Electrode
- Details
- Category: Tungsten Information
- Published on Thursday, 03 December 2015 16:19
Tungsten copper electrodes are usually activated sintered by porous tungsten skeleton pre-sintering or mixed powder with active elements. Generally, due to tungsten does not dissolved in the liquid phase copper, which lead to the porosity of high tungsten content of the composite material and has a bad effect on the process of densification. In order to reduce the porosity, we can take advantage of additives which dissolves tungsten.
Adding activate element to promote tungsten dissolution, and of the solid phase sintering and liquid phase diffusion densification during sintering tungsten particles dissolved in the liquid phase precipitation, round and accumulation, to obtain a high density. Its addition can remarkably improve the material properties, reduce the sintering temperature and shortening the sintering time. However, the addition of these elements will be activated in a certain extent, reduce the thermal conductivity and electrical conductivity of tungsten copper electrode. Therefore, this kind of method is suitable for the occasions, which has lower demands of thermal conductivity and electrical conductivity.

| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Annealing Process Effect on Tungsten Copper Electrode Properties II
- Details
- Category: Tungsten Information
- Published on Thursday, 03 December 2015 16:17
Some experiments shows that annealing process has a great influence on the electrical conductivity of tungsten copper electrode, there is a table of the electrical conductivity of W-25Cu tungsten copper electrode at different temperature in the part one.
The table shows that when the annealing temperature reaches 800 ℃, the electrical conductivity of tungsten copper electrode is the highest, after which the temperature continues to rise, the conductivity decreased. Theoretically, this is due to the elimination of internal stress and the Cu phase recrystallization.
On the one hand, when tungsten skeleton is starting cooling process after infiltrated at high temperature, it produce large internal stress because of the a great difference between the coefficient of thermal expansion of tungsten (W) and copper (Cu), which affects the electrical conductivity of tungsten copper electrodes. On the other hand, copper phase recrystallized at 400 ℃, and when the temperature rises to 800 ℃, copper atom lattice distortion reduced, the crystal defects supplemented and improved conduction mechanism of Cu is further reflected. However, with further increase of annealing temperature, changes in Cu phase grain boundaries will gradually slow, the impact on the conductivity becomes negligible.
More infomation about annealing process effect on tungsten copper electrode properties, click here:
http://news.chinatungsten.com/en/tungsten-information/80963-ti-10443
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com