Oxidation Mechanisms of W-Based Alloys
- Details
- Category: Tungsten's News
- Published on Sunday, 30 April 2023 14:55
The oxidation mechanisms and failure mechanisms of W-based alloys indicate that the oxidation process can be divided into two distinct phases. The rate of oxide film formation and the stability of the structure are the key factors affecting the oxidation resistance of W-Cr alloys. the Si and Cr elements are easily oxidized to form a stable protective film. Therefore, the oxidation properties of W-Si-based alloys and W-Cr-based alloys have been extensively investigated.
For W-Si binary alloys, the oxidation resistance increases with increasing Si content within a certain range. This is due to the fact that the relatively high Si content facilitates the rapid formation of oxide films on the alloy surface during the oxidation process. However, too high a Si content reduces the toughness of the alloy. Among the W-Si ternary alloys, the W-Si-Cr alloy has the best oxidation resistance. Increasing the thickness of WSi10Cr10 thin film alloy is beneficial to promote the formation of Cr2O3 protective film on its surface.
For W-Cr binary alloys, the oxidation resistance not only depends on the Cr content but also is affected by the surface phase distribution. The addition of an appropriate amount of Cr can make the surface structure of the alloy more uniform and compact, which is conducive to the production of continuous and uniform oxide film on the alloy surface during the oxidation mechanisms. For the W-Cr-Ti ternary alloy, the Ti element is oxidized into Ti2CrO5 oxide film during oxidation, which can inhibit the diffusion of O atoms into the alloy.
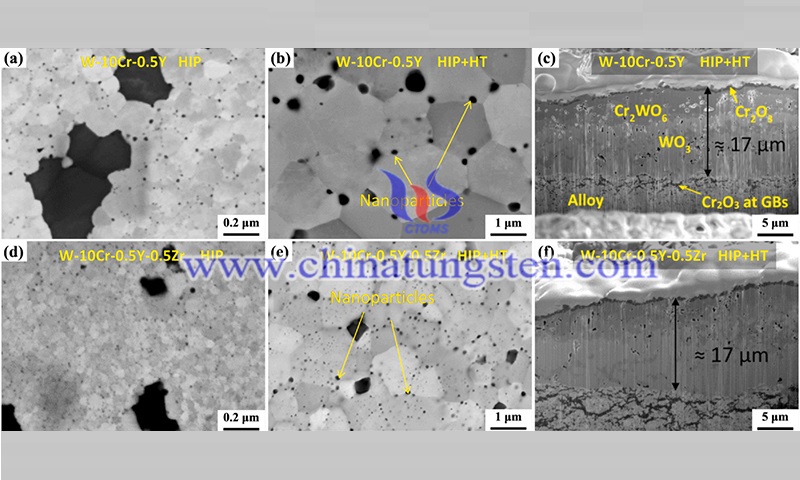
For the W-Cr-Y ternary alloy, the Y element combined with oxygen to form Y2O3 particles can effectively inhibit the growth of grains on the surface of the alloy and improve the high-temperature strength of the alloy. In addition, the Y2W3O12 particles produced during oxidation can stabilize the morphology of the Cr2O3 oxide film and enhance the self-passivation effect of the alloy.
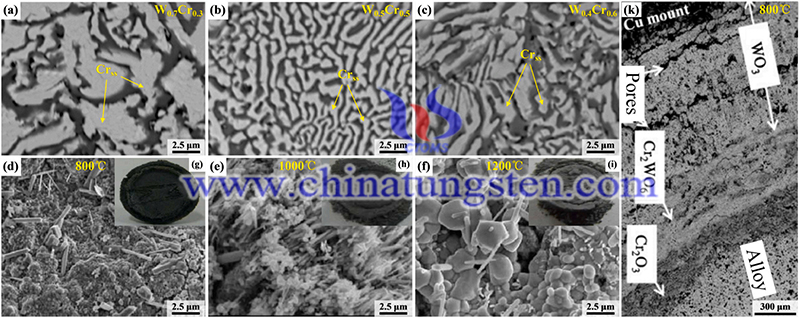
For the W-Cr-Nb ternary alloy, the Nb element can combine with Cr to form the second phase Cr2Nb, which can refine the alloy composition and increase the density of the alloy, and the oxidation resistance of the alloy increases with the increase of Cr/Cr2Nb. For the W-Cr-Zr ternary alloy, the Zr element can not only act as a nucleation center in the oxidation process but also act as a diffusion barrier for the Cr cations to reduce the degree of Cr oxidation. It is noteworthy that the oxidation resistance of W-10Cr-0.5Y-0.5Zr alloy is not significantly improved compared with that of W-10Cr-0.5Y alloy.
Reference: Fu T, Cui K, Zhang Y, et al. Oxidation protection of tungsten alloys for nuclear fusion applications: A comprehensive review[J]. Journal of Alloys and Compounds, 2021, 884: 161057.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com