Antioxidant Coating for W-Based Alloys
- Details
- Category: Tungsten's News
- Published on Sunday, 30 April 2023 15:16
Surface antioxidant coating technology for W-based alloys is an important measure for the oxidation protection of material surfaces and has been widely used for the oxidation protection of refractory metals. Among them, halide-activated packet cementation (HAPC), chemical vapor deposition (CVD) technology, and hot dipping silicification (HDS) technology have won the favor of more researchers in the oxidation protection of W-based materials due to their excellent process conditions.
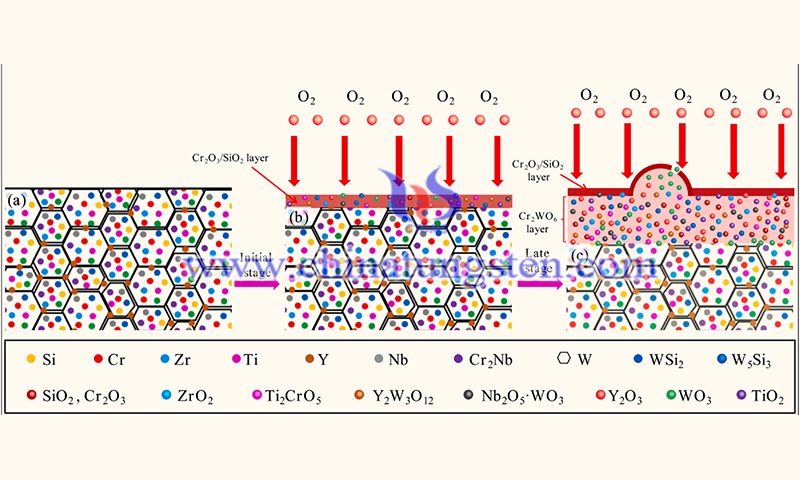
The cross-sectional morphology of the HAPC-coated coatings shows a typical columnar crystal structure perpendicular to the W substrate, and EPMA chemical analysis indicates that the columnar crystals are in the WSi2 phase. Due to the mismatch in the coefficient of thermal expansion (CTE) between the coating and the substrate, a large number of longitudinal cracks were observed inside the coating.
Moreover, a thin transition layer between the coating and the substrate is observed in XTEM bright-field images. EDS analysis shows that the transition layer is W5Si3 phase, as shown in Fig. 24 (b). This is due to the fact that the concentration of Si element gradually decreases during the diffusion into the coating. The same results were also confirmed in the study of Alam et al. as shown in Fig. 24 (c). In addition, the square of coating thickness is proportional to the embedding time, which means that the growth of coating conforms to parabolic law. The growth rate of embedded WSi2 coating at 1100 °C is 20.5 µm2·h−1.
The surface and cross-sectional morphology of the oxidized HAPC coating for W-based alloys showed some micro cracks on the surface of the oxidized coating due to the release of thermal stresses during the oxidation process. In addition, longitudinal cracks penetrating the entire cross-section of the antioxidant coating were observed, and the same composition as the oxide layer was detected inside the cracks.
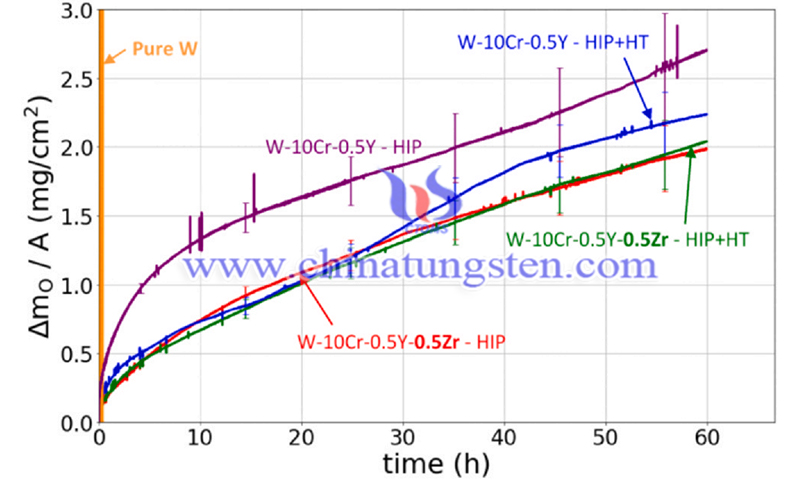
After cyclic oxidation at 1100 °C for 6 h, a small number of needle-like particles were observed on the surface of the coating. SEM-EDAX analysis showed that these particles consisted of W and O, while the oxide layer was mainly composed of SiO2 and WO3. In addition, the behavior of the coatings was investigated in cyclic oxidation and isothermal oxidation at 1300 °C, respectively. The surface of the coating after 5 h of cyclic oxidation was very rough, and SiO2 particles were mixed with a large number of WO3 residual particles. The mass loss per unit area of the coating was 145.11 mg.cm-2. The thickness of the oxidized layer was up to 20 µm and large longitudinal cracks penetrated the entire coating section.
However, only a thin oxide layer and some cracks were observed on the surface of the coating after isothermal oxidation. The mass loss per unit area of the coating was only 10.14 mg·cm−2. The stress concentration was more likely to occur at the edges of the samples during cyclic oxidation compared to isothermal oxidation, which led to the formation and further expansion of cracks. Also, a large amount of chalking and flaking was observed at the edges of the samples during cyclic oxidation. Therefore, the degree of damage of cyclic oxidation is much higher than that of isothermal oxidation.
Reference: Fu T, Cui K, Zhang Y, et al. Oxidation protection of tungsten alloys for nuclear fusion applications: A comprehensive review[J]. Journal of Alloys and Compounds, 2021, 884: 161057.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com