High-Purity Tungsten Carbide Fabricate Using Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Wednesday, 25 August 2021 01:39
Tungsten carbide (WC) has several excellent properties, including high melting point (2600–2850 °C), great hardness, high fracture toughness, high electrical and thermal conductivities, which make it become a promising material in various fields. Considering these excellent properties, WC has been widely used as cemented tungsten carbide materials in the tool industry to produce cutting, mining tools, drilling tools and general wear parts.
Several methods have been proposed to prepare tungsten carbides, including mechanical alloying, solid state metathesis, thermo-chemical reaction, gas-solid reaction, and combustion synthesis. However, it is hard to produce ultrafine WC powders by these methods mentioned above. Thus, carbothermal reduction method has been applied in the synthesis of tungsten carbide. High-purity tungsten carbide has been successfully fabricate using tungsten trioxide (WO3) as raw material. The synthesis of high-purity WC is as below:

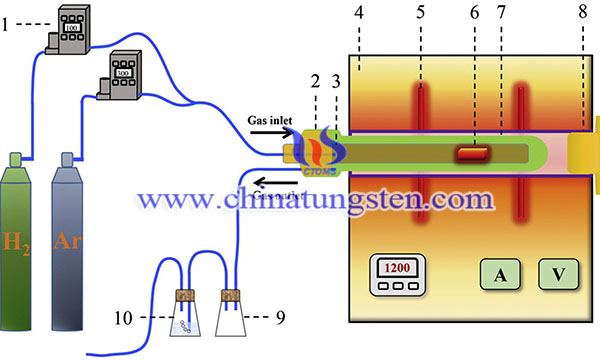
At the carbothermic reduction stage, 10 g mixed powders were placed into an alumina boat (45 mm length, 15 mm width, and 20 mm height), which was accommodated into the constant-temperature zone of a vertical tube furnace using MoSi2 rods as the heating elements. The temperature measured by a type-B thermocouple (Pt-6 pct Rh/Pt-30 pct Rh) was accurately controlled within ±1 K by a PID controller. The schematic diagram of the experimental apparatus is described and shown in Fig. 2. The furnace temperature was raised to the desired value at a heating rate of 5 °C/min and held for different time. After that, the sample was gradually cooled down to room temperature before being taken out from the furnace. During the entire process, Ar gas was introduced into the furnace with a flow rate of 400 mL/min. The carbon content of the sample after carbothermic reduction was detected. Then, an appropriate amount of carbon black was added to the sample according to the difference of carbon content between the sample and pure WC. At the following carburization stage, the mixture was put into the constant temperature zone, and then the temperature of the furnace was heated to the 1200 °C at a heating rate of 5 °C/min and held for 6 h to start the carburization reaction under a hydrogen flow rate of 20 mL/min. At the carbothermic reduction stage, 10 g mixed powders were placed into an alumina boat (45 mm length, 15 mm width, and 20 mm height), which was accommodated into the constant-temperature zone of a vertical tube furnace using MoSi2 rods as the heating elements. The temperature measured by a type-B thermocouple (Pt-6 pct Rh/Pt-30 pct Rh) was accurately controlled within ±1 K by a PID controller. The schematic diagram of the experimental apparatus is described and shown in the picture. The furnace temperature was raised to the desired value at a heating rate of 5 °C/min and held for different time. After that, the sample was gradually cooled down to room temperature before being taken out from the furnace. During the entire process, Ar gas was introduced into the furnace with a flow rate of 400 mL/min. The carbon content of the sample after carbothermic reduction was detected. Then, an appropriate amount of carbon black was added to the sample according to the difference of carbon content between the sample and pure WC. At the following carburization stage, the mixture was put into the constant temperature zone, and then the temperature of the furnace was heated to the 1200 °C at a heating rate of 5 °C/min and held for 6 h to start the carburization reaction under a hydrogen flow rate of 20 mL/min.
In summary, high-purity tungsten carbide has been fabricate using tungsten trioxide in a carbothermic reduction route. It can be concluded from experimental results that particle size of WC increased with the increased of temperature, but decreased with increased of C/WO3 molar ratio. When the C/WO3 molar ratio is 2.7–3.5, the single-phase WC with a size of 178–825 nm can be obtained after further carbonization at 1200 °C. Carbon content of final products is very close to the theoretical value of tungsten carbide.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com