Strategies for Improving the PEC Water Splitting Performance of WO3
- Details
- Category: Tungsten Information
- Published on Wednesday, 25 August 2021 01:26
Hydrogen fuel could play a key role in future sustainable energy systems as demand for energy is globally increasing. Moreover, an environment friendly energy resources are in urgent need to replace the current fossil fuel energy systems. Among the available energy sources, hydrogen possesses significantly high energy density compared to conventional fossil fuels and hence emerges as one of the ideal replacements to fossil fuels.
Applying a photoelectrochemical (PEC) cell to drive water splitting into O2 and H2 provides a feasible way to achieve the storage and conversion of solar energy. Hence, photoelectrochemical (PEC) water splitting has become one of the essential and rapidly developing research areas due to its renewable and sustainable route of producing hydrogen and oxygen.
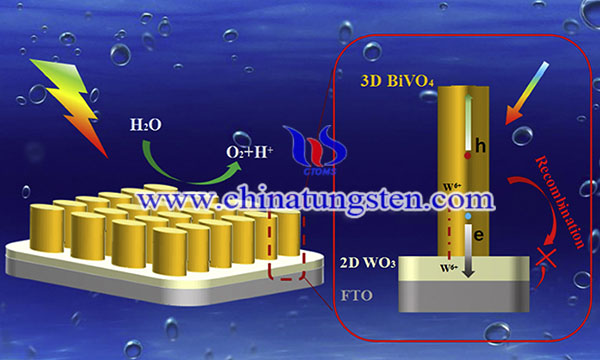
In an ideal PEC cell, an n-type semiconductor and a p-type semiconductor need to be connected in series as anode and cathode respectively, to achieve water splitting under visible light driving. Tungsten trioxide (WO3, Eg ≈ 2.7 eV) is an n type semiconductor has been applied in PEC water splitting due to its excellent photosensitivity, strong oxidation capacity and corrosion resistance. However, WO3 also suffers from low photocatalytic efficiency. Here we provide some common strategies for improving the PEC water splitting performance of WO3.
1) Controlling the morphology of WO3
Commonly, semiconductors with the morphologies of nanowire, nanoflake, and mesoporous are beneficial for the transfer of photogenerated holes from the bulk to the surface.
2) Introducing defects
Defects in materials can act as active sites in catalytic applications. It is reported that the effects of physical defects, such as microsized holes or cracks, and chemical defects, such as oxygen vacancies, on the PEC water splitting performance of WO3. The results showed that physical defects inside the film increased the resistance to charge transfer and resulted in a higher recombination rate, which inhibited the photocurrent generation. Chemical defects yielded an increased adsorption of OH groups on the film surface and enhanced the PEC efficiency.
3) Constructing a heterojunction
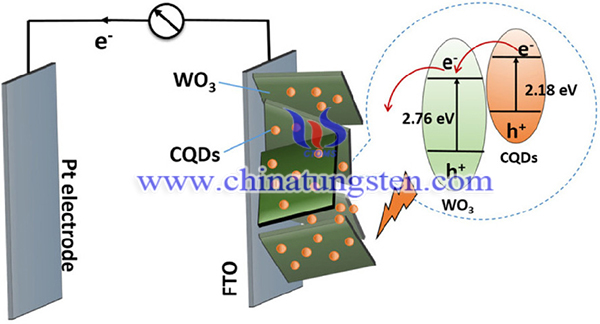
Construction of a heterojunction is an effective method of improving the separation efficiency of the photogenerated charges or sometimes increasing the light absorption efficiency. Herein, the combination of WO3 with another semiconductor as the photoanode for PEC water oxidation is reviewed. Titanium dioxide (TiO2) is a widely used photocatalyst. It has the advantage of being stable and low cost. Coupling TiO2 with WO3 to form a WO3/TiO2 heterojunction photoanode is reported to improve the PEC water splitting performance. Other examples of WO3 with a heterojunction structure include WO3/BiVO4 heterojunction, WO3/Fe2O3 heterojunction, WO3/carbon quantum dots (CQDs) heterojunction. WO3/Sb2S3 heterojunction WO3/CuO heterojunction, WO3/ZnWO4 heterojunction, WO3/CuWO4 heterojunction, WO3/Cr2O3 heterojunction, WO3/conducting polymer heterojunction, and rGO-WO3 composite. There are the proved to effective to enhance the PEC water oxidation.
4) Loading a cocatalyst
Both heterogeneous and homogeneous cocatalysts have been extensively studied for the photocatalytic and photoelectrocatalytic water splitting process.
In short, tungsten trioxide (WO3, Eg ≈ 2.7 eV) is an n type semiconductor has been applied in PEC water splitting but has the drawbacks of low photoelectrocatalytic efficiency. Some strategies for improving the PEC water splitting performance of WO3 has been provided above.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com