Carbon Dioxide Preparing Pyrochlor Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Tuesday, 02 February 2016 16:32
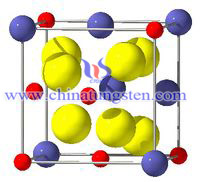 Pyrochlore type structure is also known as yellow-green stone structure or pyrochlore structure. Formula A2B2O7, (BO6) octahedra are connected by common oxygen vertex along the three-dimensional framework that is formed. (BO6) slightly distorted octahedral shape; the ion is located in the gap BO6 skeleton. Many complex oxides having such structure, such as coke cadmium ,niobate Cd2Nb2O7, tantalum acid pyrophosphate cadmium Cd2Ta2O7, coke lead niobate Pb2Nb2O7, coke cerium zirconate Ce2Zr2O7 like. Many of these oxides are important ferroelectrics, which are widely used in electronic ceramics.
Pyrochlore type structure is also known as yellow-green stone structure or pyrochlore structure. Formula A2B2O7, (BO6) octahedra are connected by common oxygen vertex along the three-dimensional framework that is formed. (BO6) slightly distorted octahedral shape; the ion is located in the gap BO6 skeleton. Many complex oxides having such structure, such as coke cadmium ,niobate Cd2Nb2O7, tantalum acid pyrophosphate cadmium Cd2Ta2O7, coke lead niobate Pb2Nb2O7, coke cerium zirconate Ce2Zr2O7 like. Many of these oxides are important ferroelectrics, which are widely used in electronic ceramics.
Since the pH of the solution is important for the preparation of tungsten trioxide pyrochlore type process and in the alkaline environment of the powder can be prepared, therefore, the following describes carbon dioxide as an additive to prepare pyrochlore type tungsten trioxide. Taking carbon dioxide as additive to prepare pyrochlore-type structure of the tungsten trioxide. First, preparing 100g / L of sodium tungstate solution, taking the carbon dioxide into the solution until the pH value of the sodium tungstate solution decreases to 7.34, and then placing the carbon dioxide treatment after the sodium tungstate solution in an autoclave 100mL at 200 ℃, reflecting it at 260 ℃ conditions about 24h, removing the solution, and washing the resulting product with deionized water 2-3 times, drying it t at 60 ℃, finishing pyrochlor tungsten trioxide.
As we can see from the entire manufacturing process, though the carbon dioxide presents in the solution, the entire system or hot water is alkaline under alkaline conditions, it is also can be prepared pyrochlore type tungsten trioxide.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Adding Carbon Dioxide to Produce Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Monday, 01 February 2016 17:21
There are two steps in the production of Ammonium paratungstate (APT) which are Preparation of pure ammonium tungstate solution and Crystallization of ammonium paratungstate. And ways to prepare pure ammonium tungstate are as follows: ion exchange, solvent extraction and classical method. A simple method which can be used in the process of alkali recycle, and using carbon dioxide reacts with sodium-ammonium salt solution to prepare ammonium paratungstate directly is put forward in this paper.
Steps like bellows:
1. Adding ammonia or ammonium salts or solution to sodium-ammonium salt solution of tungsten trioxide concentration about 15~450g/L;
2. Adding carbon dioxide to sodium-ammonium salt solution, and heating the temperature up to 20~100℃, then keep for 1~72 hours to generate ammonium paratungstate crystal slurry;
3. Separate the slurry, the solution after separation return to the tungsten concentrate decomposition process;
4. Using ammonium solution to wash the solid obtained in step 3 at temperature 20~100℃, then washed with water, and then get ammonium paratungstate.
The advantages are like bellows:
1. Realize the goal of Alkali circulation to reduce the environmental pollution in the process of tungsten metallurgy;
2. Alkali neutralization and ammonium paratungstate crystallization complete in one step which simplify the processing;
3. Low energy consumption and investment.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
High Efficiency Molybdenum Removal for Producing Ammonium Paratungstate––Guanidine-Salt-separation method
- Details
- Category: Tungsten Information
- Published on Monday, 01 February 2016 17:18
 Guanidine-Salt -separation method, the method for producing ammonium paratungstate, which can effectively remove molybdenum and increase the crystallization rate of the product.
Guanidine-Salt -separation method, the method for producing ammonium paratungstate, which can effectively remove molybdenum and increase the crystallization rate of the product.
Steps:
1. Raw materials and catalyst, environmental selection;
Raw materials: industrial ammonium tungstate solution after removal of silicon; catalyst: silicon dioxide (SiO2) or silica; pH value at 7.0-8.0
2. Heating to precipitation and then filtrate the catalyst;
3. Adding carbamamidine or derivative of carbamamidine as a precipitating agent to precipitating tungsten (W) as a salt deposit (precipitation rate reaches 98% or more), and molybdenum (Mo) removed in the filtrate (the removal rate for molybdenum reaches 95% or more);
4. Filter and wash the precipitate with cold water for several times;
5. Adding precipitate to ammonia to form ammonium tungstate, while the precipitation agent exist sin a solid state;
6. Heating the ammonium tungstate to 80-100℃ to generate ammonium paratungstate crystal, the crystallization rate can reach 95%.
Noted: Controlling the pH value of precipitation in the process is very important, partly molybdenum will be precipitated when pH value is too low, thus reduce the effect of removing molybdenum; tungsten precipitation will be not completely when pH value is too high, and it will reduce the recovery rate of tungsten.
The advantages are as bellows: effectively remove molybdenum, crystallization rate of ammonium paratungstate can be increased by nearly 20% and reduce the cost, simplify the operation, compared with the traditional production process.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
The Special Micro-structure of Tungsten Copper Electrode by Microwave Infiltration
- Details
- Category: Tungsten Information
- Published on Monday, 01 February 2016 17:06
Microwave Infiltration is a new process that combines microwave sintering and infiltration process. Compared with the microstructure of conventional sintering process infiltrated tungsten copper alloy electrodes can find significant differences. For tungsten copper alloy electrode samples of the same composition, at the same sintering parameters using microwave infiltrated samples can be found in a large number of rod-shaped tungsten grain elongated and showed the existence of, and the kind of tungsten rod having a directional grain growth trend. The tungsten grains infiltration under conventional sintering process is more loose and uneven distribution, mostly near-spherical and ovoid, in the organization not only has a large tungsten grain size, there are a lot of smaller grain size tungsten grains, but these tungsten grains merger does not occur at the same time there is no directional trend growth.
Analyzing the experimental data and scanning electron microscope picture, we can find that rod-like tungsten (W) grain is obvious when copper (Cu) content is relatively low. And with the increasing of copper content, tungsten grains are merging and growing, which the directional growth trend is more obvious. In addition, the orientation of the tungsten particle growth law is not certain rules to follow, there is a radial, like rivers, swirling and so irregular shape, which has become some new research directions of scholars and researchers.
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Effect of Cu Content on Tungsten Copper Electrode Micro-structure
- Details
- Category: Tungsten Information
- Published on Monday, 01 February 2016 17:04
We can observe that sample with higher Cu content has smaller average granularity at the same sintering temperature and holding temperature by analyzing the picture of tungsten copper electrode micro-structure scanning electron microscope (SEM) after infiltration. This is due to increased copper content of the tungsten particles beneficial rearrangement, but also to promote the role of liquid copper segregation, so that the tissue sample composition is unevenly distributed. While the liquid copper segregation would to some extent caused by a corresponding tungsten particles contact with each other, which also reached a solid-phase sintering conditions will lead to further agglomerated tungsten particles, especially evidently in tungsten copper W-50Cu electrode sample. The following is a microwave Infiltration prepared tungsten copper alloy electrode scanning electron microscopy (SEM) photograph, showing the tungsten (W) grains agglomeration is a more clear and intuitive:
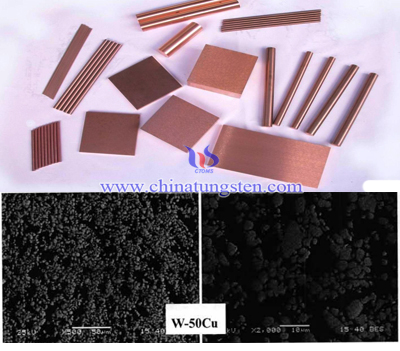
Compared with the micro-structure of tungsten copper electrode in conventional infiltration, microwave infiltration has higher heating rate, which is beneficial for copper liquid flowing and uniform distribution, so it has better uniformity and stability of structure. In addition, in the lower copper content, microwave Infiltration tungsten copper alloy electrode samples may also have a more clear view of the elongated rod dispersed tungsten grains W grow; low Cu content of tungsten copper electrode by conventional infiltration has inhomogeneous distribution of tungsten (W) particles, and like a nearly spherical or oval, which not only has tungsten particles with large granularity, but also has the small granularity. Accordingly, we can also make further concluded that the adverse effects of conventional infiltration microstructure produced for its relatively slow rate of temperature rise there is a certain correlation (conventional infiltration heating rate is generally 5 ℃ / min, while the microwave Infiltration the heating rate is generally 30 ℃ / min).
| Tungsten Copper Supplier: Chinatungsten Online tungsten-copper.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Sensitizing Tungsten Trioxide Surface Enhancing Photoelectric Conversion
- Details
- Category: Tungsten Information
- Published on Monday, 01 February 2016 16:32
 The use of surface sensitized method to improve the photoelectric conversion efficiency. The material must be supported on tungsten trioxide (WO3) semiconductor surface, thereby improving the light absorption and conversion capabilities. The surface sensitized principle is quite similar to semiconductor composite, but the light absorption by the conversion of primary tungsten oxide material turn into photosensitizer and photosensitizer, which must meet two requirements: (1) the band gap must is less than WO3; (2) the position of conduction band generally lost WO3 conduction band. It has now been found to meet the conditions by using a semiconductor mainly Fe2O3, Cu2O, CdS, etc.
The use of surface sensitized method to improve the photoelectric conversion efficiency. The material must be supported on tungsten trioxide (WO3) semiconductor surface, thereby improving the light absorption and conversion capabilities. The surface sensitized principle is quite similar to semiconductor composite, but the light absorption by the conversion of primary tungsten oxide material turn into photosensitizer and photosensitizer, which must meet two requirements: (1) the band gap must is less than WO3; (2) the position of conduction band generally lost WO3 conduction band. It has now been found to meet the conditions by using a semiconductor mainly Fe2O3, Cu2O, CdS, etc.
Semiconductor composite improves the photoelectric conversion performance of WO3. Semiconductor composite are two or more semiconductors by physical or chemical combination, which is relatively common, particular way of effectively to enhance the performance of the material. In order to improve the photoelectric conversion performance, composite machine generally is used as metal oxide, in short, which enables the photo-generated electrons or holes by compound semiconductors. It is gathered in two semiconductor conduction band or the valence band, so that electron and hole separated, thus improving the photoelectric conversion efficiency. In order to enhance the photoelectric conversion efficiency of the semiconductor, the use of metal oxides also using probability electron transfer media, which can improve the speed of migration of electrons in semiconductor material while reducing green light to stay in the complex.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Increasing Tungsten Trioxide Surface Energy Improving Photoelectric Conversion
- Details
- Category: Tungsten Information
- Published on Monday, 01 February 2016 16:21
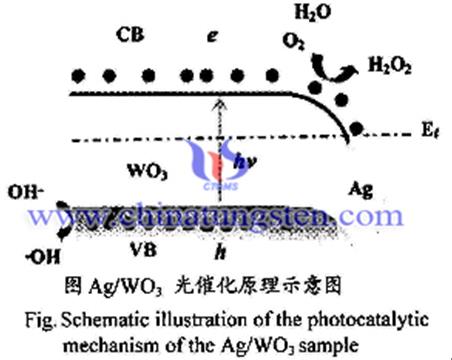
 Improving photoelectric properties by taking precious metals surface as loading energy of tungsten trioxide (WO3) . The more common ways for noble metal are sputtering, deposition light, liquid adsorption, hydrogen reduction and the boron compound pulse electrodeposition method. And many scholars have reported the use of more precious metals deposited on WO3 surface together or sequentially deposited using hierarchical manner. Scholars found that when the load is metal Ag, optical and electrical properties of WO3 most is obvious, when the light irradiating on the surface of WO3, electron energy will start to give the valence band to the conduction band, and then migrate to the Ag nanoparticles on the light-generated electron-rich, lower electron - hole recombination probability.
Improving photoelectric properties by taking precious metals surface as loading energy of tungsten trioxide (WO3) . The more common ways for noble metal are sputtering, deposition light, liquid adsorption, hydrogen reduction and the boron compound pulse electrodeposition method. And many scholars have reported the use of more precious metals deposited on WO3 surface together or sequentially deposited using hierarchical manner. Scholars found that when the load is metal Ag, optical and electrical properties of WO3 most is obvious, when the light irradiating on the surface of WO3, electron energy will start to give the valence band to the conduction band, and then migrate to the Ag nanoparticles on the light-generated electron-rich, lower electron - hole recombination probability.
Dopant ions can increase the photoelectric properties of WO3. Ion doping is mainly accessed through the cation and anion inside the lattice to WO3, WO3 semiconductor substitution of W + W O2- ions or oxygen ions to affect the electron excitation and electron - hole separation. The study shows doping Cr, Mo equivalent of these metal replacement lattice W atom, not only is small on the geometry of the lattice, but also the bottom of the conduction band reduce the band gap; when the doping Ti, Zr, Hf, W is less than the valence state of the metal . Oxygen vacancies, oxygen vacancies are formed. The W atoms will cause the bottom of the conduction band of the shift because the band gap decreased from doping on the whole.
| Tungsten Oxide Supplier: Chinatungsten Online www.tungsten-oxide.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Intermediate Compound of Tungsten - Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Friday, 29 January 2016 19:45
Ammonium paratungstate (APT) is a primary product of tungsten, which is an important raw material for the manufacture of tungsten products and hard alloy. Also it’s the most common tungsten compound, and widely used in tungsten smelting. The quality of APT determines properties and quality of tungsten products in a large extent. WO3, blue tungsten oxide, tungsten acid, ammonium metatungstate can be generated through partly or completely heating. APT is a white transparent crystal with good loose and mobility. APT will begin to loose ammonia when heated in the air up to 60℃, begin to dehydration at 100℃, and begin to turn into yellow tungsten oxide at 450℃. APT is completely converted into blue tungsten oxide When heated to 300℃ in hydrogen, and turned into violet tungsten at 400℃, trioxide at 500 to 600℃, and turned into tungsten powder when the temperature reach to 600~900℃.
APT crystal powder can be prepared by evaporation of ammonium tungstate solution, the temperature supposed to be generally controlled at more than 50℃, and its particle size and composition changes with the temperature:
1. Evaporation and crystallization 80~100℃, APT present as flake crystal with 5 molecules of crystal water;
2. when the temperature is below 50℃, APT present as fine white needle crystal with 11 molecules of crystal water.
The number of water in APT crystal decided by the production conditions, and the solubility increased with temperature increasing. Heating the APT with high impurities content to get decomposition products, and then dissolve in ammonia solution, and heated at the same time, evaporate and crystallize, round cycle 1~2 times or more, then get high purity APT.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Prepare Phosphato-Tungstic Acid from Ammonium Paratungstate
- Details
- Category: Tungsten Information
- Published on Friday, 29 January 2016 19:42
A method for preparing phosphato-tungstic acid by using ammonium paratungstate as raw material is proposed in this paper. The principle is: ammonium paratungstate reacts with inorganic acid to get a reactive acid precipitation, and then contacts with phosphoric acid to obtain phosphato-tungstic acid solution, crystallize and we get phosphato-tungstic acid crystal.
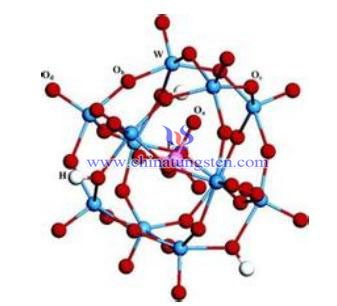
Specific steps are as follows:
1. Ammonium paratungstate reacts with inorganic acid to get a reactive acid precipitation;
Inorganic acid can be hydrochloric, sulfuric, nitric or hydrochloric acid, sulfuric acid and nitric acid can mix at any volume ratio, the appropriate concentration of 0.2 mol/L~6.0mol/L; the amount is optional, as long as active precipitation of phosphato-tungstic acid is able to generated; the reasonable temperature is10 ~ 40℃; reaction time for 2~5 hours;
2. Active precipitation of phosphato-tungstic acid reacts with excessive phosphoric acid solution, and get phosphato-tungstic acid solution;
3. Precipitate phosphate acid from the solution, drying and crystallizing to get the crystal.
| APT Supplier: Chinatungsten Online ammonium-paratungstate.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News&Tungsten Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |
Tungsten Wire Production Processes
- Details
- Category: Tungsten Information
- Published on Friday, 29 January 2016 17:56
Tungsten wire resistivity is 5.3 * 10 ^ -8, which has a high melting point, high resistivity, good strength, low vapor pressure and other excellent performance. Tungsten wire has many different species, including black tungsten wire, white tungsten wire, stranded tungsten wire, doped tungsten wire, tungsten rhenium wire and tungsten filament and so on. It is widely used in the field of electric light sources, the main application is used for create filament and high-speed cutting steel. On the other hand, it also can be used in the field of optical and chemical instruments.
Tungsten production processes are as follows:
1. ammonium paratungstate (APT) as a raw material, which was calcined in air at about 500 ℃ to obtain tungsten trioxide.
2. Doped a certain amount of potassium oxide hydroxide, silicon oxide and aluminum oxide into tungsten trioxide, wherein the total amount of the three types doped oxide is not more than 1%, then the powder is mixed uniformly.
3. Proceeded two-steps reduction, the first step is at about 630 ℃ restore tungsten oxide to tungsten dioxide, and then at about 820 ℃ restore tungsten dioxide to tungsten powder. The purpose two-step reduction is to make potassium which is doped into tungsten powder can fully play its role, but also two-step reduction can better control the particle size of the powder.
4. Placed restored doped tungsten powder in a special mold pressed into elongate square bars. The square bars are placed in hydrogen to electrify which is using and is using resistance heating method for sintering.
5. Swaging processing, sintered tungsten bar after swaging processing become about 3mm diameter tungsten rod, then drawing by wire-drawing die so its diameter becomes finer. Tungsten rods are drawing and lubrication by tungsten carbide wire-drawing die or diamond wire-drawing die, eventually processed into different thickness of the tungsten wire.
| Tungsten Supplier: Chinatungsten Online www.chinatungsten.com | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com