Tuning Electronic Structure of Tungsten Oxide for Hydrogen Electrolyte Reaction
- Details
- Category: Tungsten Information
- Published on Friday, 15 April 2022 12:32
Researchers at the Shanghai Institute of Microsystems and Information Technology (SIMIT), Chinese Academy of Sciences (CAS) have demonstrated that doping tungsten oxide (WO3) catalysts with appropriate cations can change the interfacial structure, surface chemical state, and bandgap values, thus improving the performance of the basic hydrogen electrolyte reaction (HER). In the experiments, cation (Ni, Co, and Fe) doped WO3 catalysts were synthesized on nickel foam. The Co-doped catalysts (Co WO) exhibited remarkable HER activity.
In this system, the nickel-based alloy-WO3 interface promotes the dissociation of water. In addition, the Co doping-induced bandgap reduction and conductivity enhancement further enhance the charge transfer capability in the HER process. The combination of metal-oxide heterostructure and cation doping endowed the catalyst with efficient interfacial active sites, excellent electrical conductivity and enhanced electron transfer kinetics, which promoted HER performance.
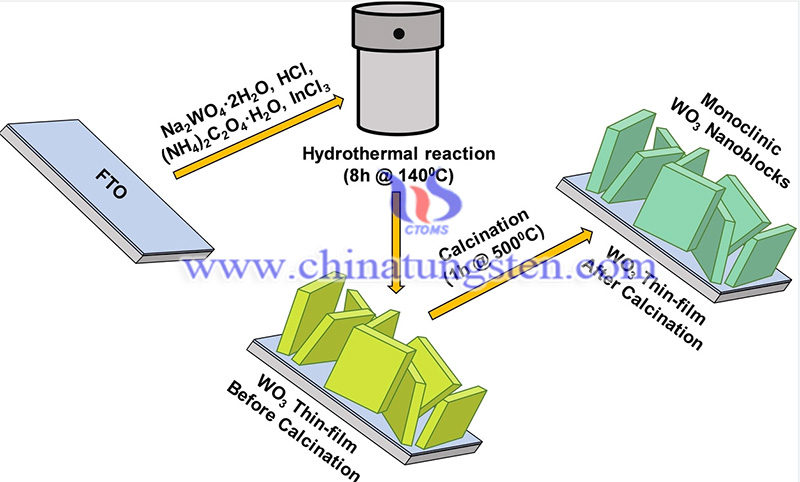
Increasing energy demand and environmental degradation have become critical issues that can be circumvented by using alternative renewable clean energy sources. Hydroelectricity, with its zero emissions and high energy density, has been widely recognized as an alternative source of renewable clean energy. Hydrogen precipitation reactions are critical to industrial hydrogen economics.
A proper electronic structure implies suitable reactant adsorption/desorption/dissociation energies, fast electron transfer kinetics, and high durability. Platinum group materials exhibit excellent hydrogen electrolyte reaction activity with proper electronic structure, but their scarcity and high cost limit the industrial applications. Therefore, much effort has been put into the exploration of efficient noble metal-free catalysts such as transition metal oxides, sulfides, carbides, nitrides, etc.
However, due to the lack of highly active sites and moderate charge transfer capability to support the catalytic reactions, these catalysts are less active in their pristine form and cannot effectively perform water separation. Therefore, various structural engineering approaches such as ion doping modulation, interfacial construction, defect creation, and single-atom construction have been explored to tailor the catalysts. Tungsten oxides with different degrees of oxygen deficiency (WO3-x) have attracted much attention in electrochemical water separation applications due to their abundant resources, good electrical conductivity, and unique defect structures.
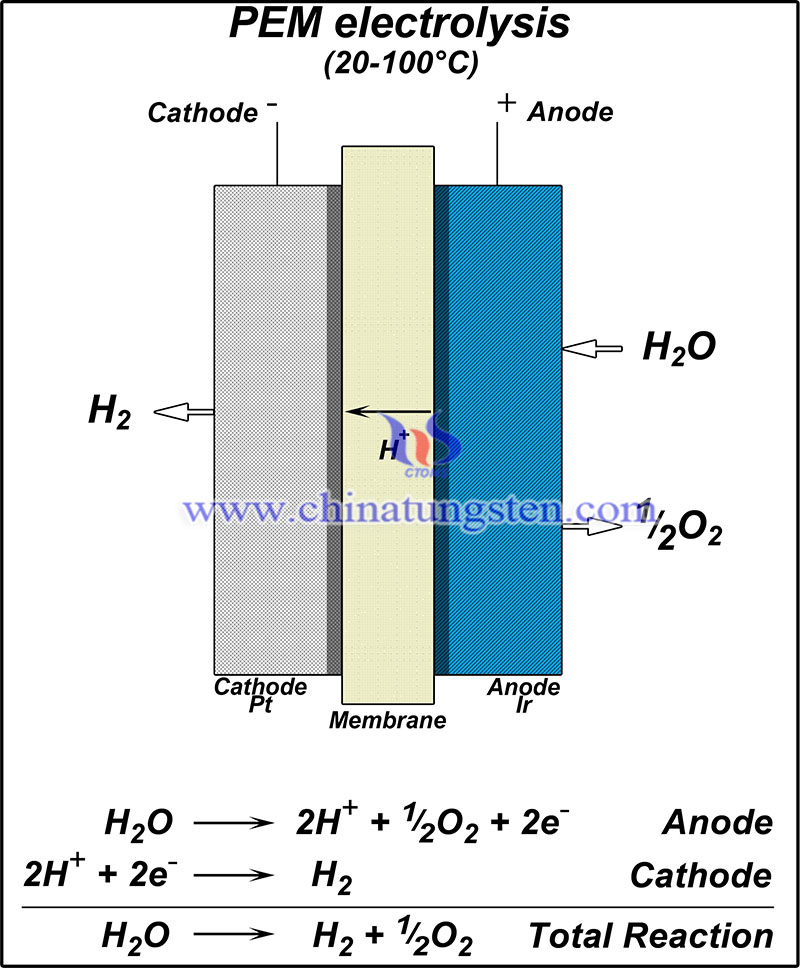
However, single-component WO3 has limitations in tuning electrochemical properties due to its lack of active sites, basic instability, and insufficient electrical conductivity. Therefore, structural engineering strategies have also been used to tune the catalytic behavior.
It is presented in this study that NiFe, NiCo, NiMo, and even NiCu can be used to modify oxides and construct dual active sites to improve water separation activity. Here, the article shows a simple strategy for designing Ni, Co, and Fe doped tungsten oxides (WO2.72 and WO2) on which to decorate Ni-based alloys. In this system, the electronic structure of the catalyst is tuned by modifying the interfacial structure, surface chemical state, and band gap value. It was shown that the metal-metal oxide interface favors the dissociation of water under alkaline conditions.
Compared with nickel-only doped tungsten oxide (WO), nickel and cobalt co-doped tungsten oxide (Co WO) has a reduced bandgap and enhanced charge transfer capability. As a result, Co WO exhibits excellent basic HER performance. The enhanced catalytic conductivity by cation doping, coupled with the metal-metal oxide interface constructed by the nickel-based alloy and WO3, simultaneously promoted the HER performance of the catalyst.
The Co WO catalyst was composed of an efficient Ni-based alloy-WO31face for water dissociation, and a decreased bandgap induced by Co doping to facilitate electron transfer. As a result, it exhibits excellent hydrogen electrolyte reaction performance in alkaline solutions. This study successfully discerned the modulating effect of the modified tungsten oxide system and provided a strategy for the design of advanced electrochemical energy materials.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com