Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials
- Details
- Category: Tungsten Information
- Published on Friday, 15 April 2022 12:27
Tungsten oxide-based nanomaterials have attracted a lot of attention for their use in building various electrochemical energy devices. In particular, electrochromic devices and optical change devices have been intensively investigated in terms of energy conservation.
A study conducted by researchers at the South China University of Technology reviews recent developments in tungsten oxide-based nanostructured materials for various applications, particularly their use with supercapacitors, lithium-ion batteries, electrochromic devices, and their bifunctional and multifunctional devices. There are also other applications such as photochromic devices, sensors, and photocatalysts with tungsten oxide-based materials.
The study titled “Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials” has been published in Nanomaterials (11). The study was carried out by Wenfang Han et al.
Energy depletion and environmental degradation have attracted increasing scientific and public attention. To slow the rate of resource depletion and improve our living conditions, there is a shift to other renewable energy sources, including solar, wind, and tidal energy. Yet under uncontrollable weather conditions, it is clearly challenging to obtain a reliable and stable supply of energy from inexhaustible sources alone.
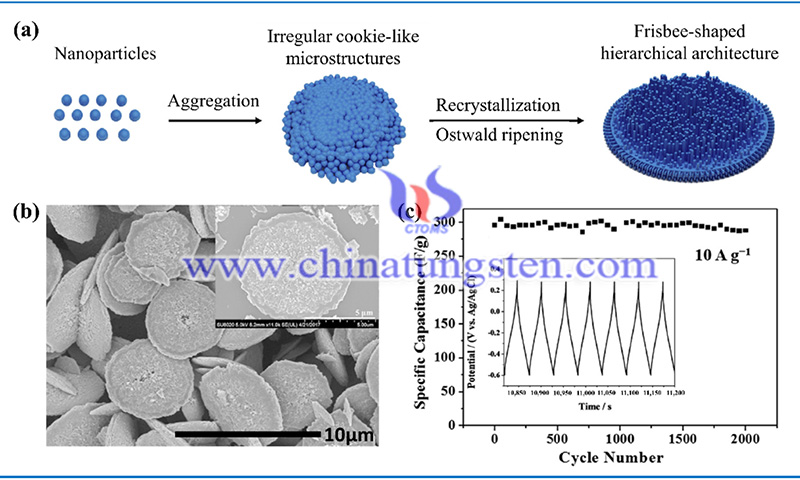
Therefore, these energy conversion systems must be used in conjunction with energy-efficient storage devices to store the converted energy. Ultracapacitors and lithium-ion batteries are two widely used types of high-efficiency energy storage devices (ESDs).
In addition, electrochromic devices (ECDs) are a well-known and efficient application to control the intensity of sunlight and the heat passing through it by changing the transmittance. Supercapacitors (SCs) are a promising energy storage device with unique advantages such as high power density, extremely long cycle life (over 105 cycles), fast charging speed (within tens of seconds), and excellent performance at low temperatures.
There are two main types of SCs, namely electric double-layer capacitors and pseudo-capacitors. The former works on the principle of charge concentration and dispersion at the interface between electrodes and electrolyte, while the latter mainly relies on Faraday reaction to work, and its double-layer capacitance contributes relatively little to the total capacitance. Usually, the capacitance of the pseudo-capacitor is higher than that of the electric double-layer capacitor. Lithium-ion batteries (LIBs) are commonly used in portable electronics and electric vehicles because of their high energy density.
Currently, the typical anode material for LIBs in electrochemical energy devices is graphite because of its low cost, stable electrochemical properties, and good structural stability. However, its theoretical specific capacity of 372 mA h g-1 is relatively low with the expanding energy consumption demand, thus limiting the further use of LIBs.
Meanwhile, transitional oxide materials, such as tin oxide, cobalt oxide, and tungsten oxide, are considered potential substitutes for graphite due to their higher specific capacity. Electrochromic (EC) materials can change their optical parameters, including reflectance, refractive index, transmittance, and emissivity, when relatively low voltages (even less than 1 V) or electric fields are applied, and the process is reversible when the polarity of the voltage or electric field is reversed.
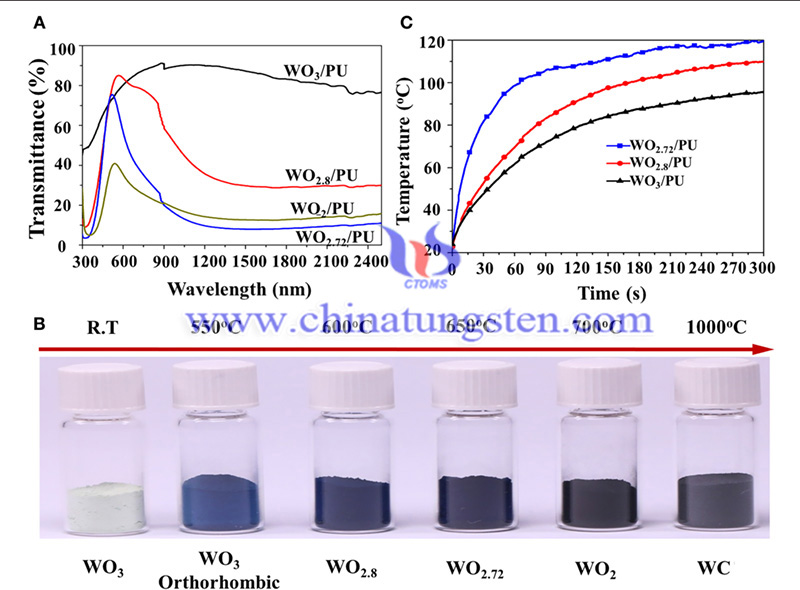
Due to their special properties, ECDs are popular in smart windows, anti-glare mirrors, display applications, and aerospace and military applications. In particular, buildings account for 40% of global energy consumption, and when they are used as smart windows, they can save significant amounts of energy due to their adjustable transmittance to sunlight.
In addition, many transition metal oxides, such as MoO3、MnO2, and WO3, can be used as electrode materials for these devices. Among them, tungsten oxide has a large energy storage capacity, which enables it to play an electrode role in ESD, and it is also the most widely studied material in the field of EC. When used as an electrode for monolithic devices, its theoretical specific capacity of 1112 F g-1 is much higher than that of the carbon electrode material normally used for double-layer capacitors, as the valence of W can vary between +2 and +6.
In addition, they offer other advantages, including high density, low cost, environmental friendliness and non-toxicity. In the field of electrochemistry, Deb discovered the first electrochemical phenomenon in tungsten oxide in the 1960s. Tungsten oxides are favored for their short switching times, impressive color changes, and electrochemical stability.
Tungsten oxides are more attractive in various fields, especially in energy storage like LIBs, SCs, and electrochromic. Based on the connection between ESD and ECD, multifunctional devices based on tungsten oxide are also widely explored. Moreover, the integration of solar energy conversion systems is a very effective way to achieve green applications. Although a lot of efforts have been made in the research of tungsten oxide, there are still many challenges that need to be addressed. The low specific capacity, poor electrical conductivity, and unsatisfactory cycling stability still need to be improved when tungsten oxides are used as electrodes for ESD.
In addition, studies on total electrostatic discharges (ECDs) of electrochemical energy devices constructed on the basis of tungsten oxide-based nanomaterials are still scarce. When they are applied to ECDs, their performance in the NIR needs more attention. The bifunctional applications mentioned in this article also have weaknesses, such as single-color, narrow voltage window, and low capacity, which limit their use in practical applications.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com