Molybdenum Improves Cathode Materials’ Performance in Li Batteries
- Details
- Category: Tungsten Information
- Published on Tuesday, 08 February 2022 23:06
A study at the University of Queensland has shown that tungsten and molybdenum (Mo) can improve the electrochemical properties of the cathode materials in Li batteries. As the driving range of electric vehicles increases, more nickel is needed to increase energy density, but this also reduces cycle stability. Tungsten and Mo improve stability by enhancing structural stability and resistance of the cathode material surface layer to electrode/electrolyte side reactions.
Nickel-cobalt-manganese ternary oxide materials (NCMs) and nickel-cobalt-aluminum materials (NCAs) are unanimously considered as ideal cathode materials for lithium-ion batteries (LIBs) due to their high energy density, good cycling performance, good safety performance, and relatively low cost.

From LCO to LiNi1/3Co1/3Mn1/3O2 (NCM111), LiNi0.5Co0.2Mn0.3O2 (NCM523), LiNi0.6Co0.2Mn0.2O2 (NCM622), LiNi0.8Co0.1Mn0.1O2 (NCM811) and ultra-high nickel (Li [ NixCoyMn1-x-y] O2 in x>0.8). Researchers are constantly exploring the capacity of lithium batteries. However, increasing the nickel content will significantly reduce other battery properties such as cycling stability (especially long-term cycling) and thermal stability.
Molybdenum has been found to play an important role in increasing the stability of cathode materials for Li batteries. For NCM622, the researchers found that 0.7 mol% Mo doping improved the cycling stability. The capacity of 1C was 203 mAh-g-1 at 4.6 V, and the Mo-doped sample had higher capacity retention than the original sample. A new study resulted that doping with Mo significantly inhibited particle crushing. The researchers further found that 1 mol% Mo doping is the optimal level.
The size of the primary particles decreases with increasing Mo doping, i.e., 800, 400, 300, 200, and 150 nm for 0, 0.5, 1, 1.5, and 2 mol% doping, respectively. Smaller particles increase the surface area and shorten the Li+ transport path. One problem may be the limited number of cycles tested (<200), since the negative effect of Mo doping on cycling stability may not occur unless long-term cycling tests are performed at high voltages.
For NCM811, researchers tried to modify the thermal stability with Mo doping, in which Mn was replaced by Mo. The DSC results proved that the exothermic reaction of the electrode/electrolyte was reduced, which is consistent with the results of NCM523. Thermal desorption spectroscopy-mass spectrometry (TDS-MS) was used to measure the gases, and O2, HF, and CO2 were detected.
Mo-doped sample showed much less oxygen release than the original sample, demonstrating the improved thermal stability. At 200-350 °C, Mo doping suppressed the phase transition from spinel to rock salt. However, the discharge capacity of the Mo-doped samples was lower than that of the pristine samples. This may be due to the high Mo content (2 and 4 mol%) since previous studies demonstrated that usually 1 mol% Mo is the optimal level.
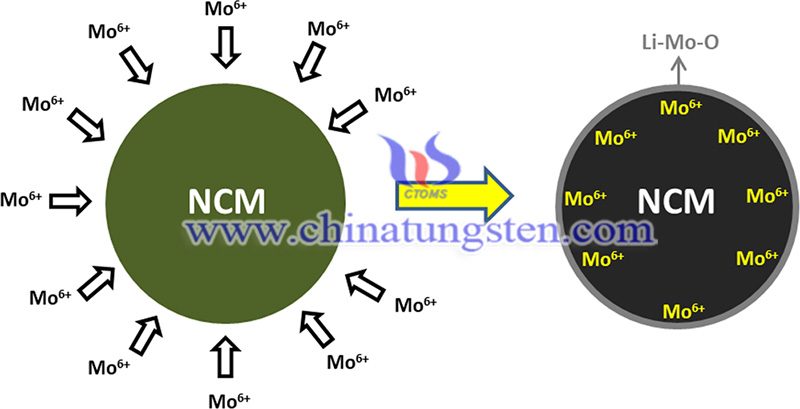
Moderate Mo doping can promote the formation of surface rock salt because of the increased concentration of Ni2+ induced by Mo6+. The rock salt formed before cycling will ease the further phase transition from lamellae to rock salt during cycling. The optimal Mo content of 1 wt% has a high capacity of 184 mAh-g-1 at 1C and capacity retention of ~92% after 100 cycles.
Mo doping (1-3 mol%) can improve the electrochemical performance considerably. The main advance is the theoretical explanation that Mo6+ would better replace Ni sites since density flooding theory (DFT) calculations demonstrate that Ni/Mo exchange shows the lowest substitution energy compared to Li, Co, and Mn. The substitution of Ni cations by Mo6+ and the increase of Ni2+ concentration can also improve the relative enrichment of Ni2+ in the local sites of the particles, inducing the rock salt phase formation.
The above discussion suggests that doping/coating with molybdenum in Li batteries cathode materials is a promising strategy to improve the cycling stability of layered cathodes, including NCM, NCA, and ultra-high nickel materials, generally in the form of lower potential polarization, smaller increases in charge transfer resistance and fewer microcracks in the cycling cathode.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com