Au-Modified Tungsten Trioxide and Its Gas Sensing to NOx
- Details
- Category: Tungsten Information
- Published on Friday, 17 September 2021 00:36
Semiconductor metal oxides (SMOs) are highly potent gas sensors for gaseous detection in terms of screening of air eminence, low expenditure on synthesis and sensing property that can be modified. The semiconductor metal oxide gas sensor is considered the most capable gas-sensing device due to its high sensitivity, fast response, low cost, and small size.
Tungsten trioxide (WO3) is a typical n-type semiconductor with a bandgap of 2.5–2.8 eV, has attracted significant attention for its distinctive sensing properties and has been regarded as a promising material for detecting various gases, including NOx, SO2, H2S, H2 and organic vapors.

Thus, the scientists modifies WO3 nanoplates with Au nanoparticles (Au NPs) to design high-performance NO-sensing materials. Au-modified tungsten trioxide is stable, sensitive, and selective to NOx and has potential applications in environmental control. The synthesis process of the Au-WO3 composite is as below:
Dispersing tungstic acid (H2WO4) in a mixture of n-octylamine and heptane, and the mixture is magnetically stirred at room temperature for 72 hours to form a tungstate-based inorganic-organic hybrid zone (THB). The molar ratio of n-octylamine to H2WO4 and the volume ratio of heptane to n-octylamine in the above mixture are both 8:1. The obtained THB was dried at room temperature for more than 3 days. A portion of the dried THB was dispersed in an aqueous HNO3 solution (~38%) and stirred magnetically for 48 hours to obtain a pale yellow suspension. The dried solid powder is H2WO4 nanosheets. Finally, the WO3 nanoplates were synthesized by calcining the H2WO4 nanoplates in air at 400 °C for 2 hours.
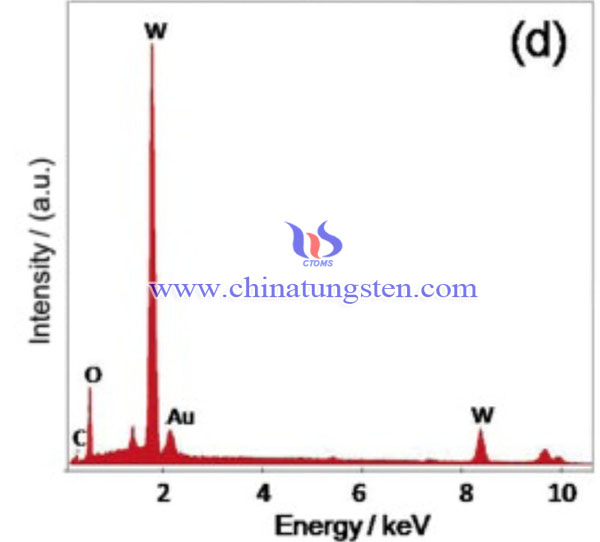
Then, 0.5 g WO3 nanosheets are dispersed in 60 mL HAuCl4 aqueous solution (~0.0016 mol L-1) under ultrasonic conditions at room temperature, and the pH value is adjusted to ~1.0 by adding 0.1 M HNO3 aqueous solution. Then, NaBH4 aqueous solution was added to the above suspension and reacted for 1 hour. The molar ratio of NaBH4 to HAuCl4 is 5:1. The suspension turned gray, indicating the formation of gold nanoparticles. Finally, the 0.5% Au-WO3 solid was collected by filtration, washed with distilled water and alcohol, and dried in a vacuum oven at 50°C for 24 hours. A similar method was used to synthesize samples with different amounts of Au NPs (ie 1% Au -WO3 and 2% Au -WO3).
In summary, Au-modified tungsten trioxide is stable, sensitive, and selective to NOx and has potential applications in environmental control. The amounts of Au NPs influence the NO-sensing performance of the Au-WO3 sensors, and the 1 wt.% Au-WO3 sample shows the best NO-sensing performance at an operation temperature range of ∼170 °C to 0.5–10 ppm NO gases. The Au-WO3 sensors have highly selective responses to NO gases among various inorganic gases (i.e., H2, SO2 and CO) and organic vapors (i.e., alcohol, acetone, methanal and benzene) when the operating temperature is lower than 200 °C. The enhancement in NO-sensing performance of the Au@plate-WO3 sensors is attributed to the functional modification of Au NPs and to the loose aggregates of WO3 nanoplates with a house-of-card structure.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com