WO3-TiO2 Nanotubes Prepared Using Ammonium Paratungstate for PEC Water Splitting
- Details
- Category: Tungsten Information
- Published on Wednesday, 25 August 2021 16:57
Global energy demand, mainly based on unsustainable fossil fuels, has grown considerably during the last decades. Moreover, the emission of green house gases had caused environmental pollution and global warming.
Hydrogen energy has become one of the most promising options to mitigate the negative impact produced on the environment by fossil fuels, such as climate change and air pollution. The main advantage of hydrogen is that it could be generated by means of renewable energy sources and water without the emission of any type of pollutant to the environment. One of the most promising and novel methods for hydrogen production is photoelectrochemical (PEC) water splitting by using solar energy.
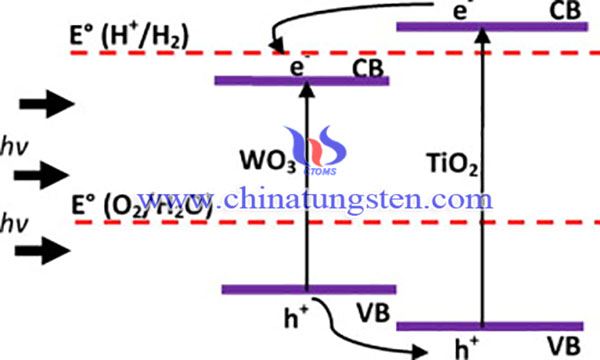
Titanium dioxide (TiO2) is the most encouraging and widely studied photoanode due to its excellent properties, such as resistance to corrosion and photocorrosion, high photochemical stability in acid and basic environments, its zero toxicity and low cost. Tungsten trioxide (WO3), like TiO2, is an n-type semiconductor with a band-gap value of roughly 2.6 eV, which indicates an absorption of approximately 12% of the solar spectrum and an absorption of the visible spectrum of up to 500 nm.
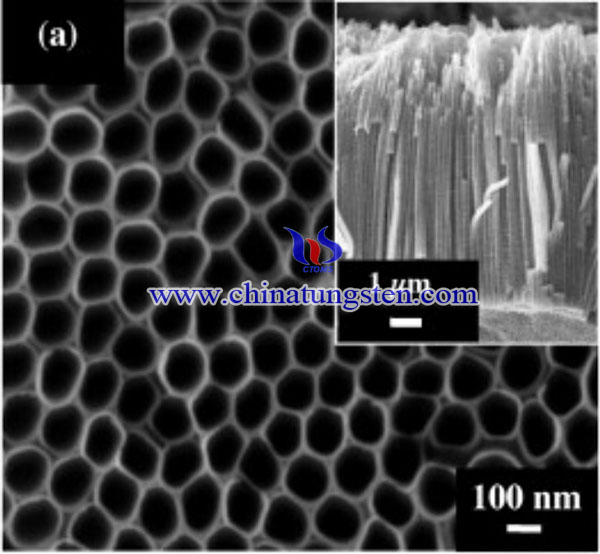
Thus, wet impregnation was used to couple TiO2 to WO3 to overcome drawbacks such as poor visible light response and recombination losses of charge carriers in PEC water-splitting applications. WO3-TiO2 nanotubes have been prepared using ammonium paratungstate for PEC water splitting, a maximum photocurrent of 2.1 mA/cm2 with a photoconversion efficiency of 5.1% was obtained. The synthesis process of WO3-TiO2 nanotubes is as below:
A 0.127 mm thick titanium (Ti) foil (99.6% purity) was used in this study. The Ti foil was cut into the desired dimension (50 mm × 10 mm) and then placed in ethylene glycol with 5 wt% ammonium fluoride (NH4F) and 5 wt% hydrogen peroxide (H2O2). During anodization, a small amount of H2O2 (≈0.5 mL) was continuously added into the electrolyte at 10 min intervals. This composition was selected because it favors the formation of well-aligned TiO2 nanotube arrays. Anodization was performed at a constant potential of 60 V with a current density of 10 mA/cm2 using a Keithley DC power supply for 1 h, with Ti foil as the anode and a platinum rod as the cathode. During anodization, air bubble was blown in the electrolyte to maintain a uniform current near the Ti electrode. The anodized Ti foils were washed with distilled water and then dried in a nitrogen (N2) stream. WO3–TiO2 nanotubes were prepared through wet impregnation using ammonium paratungstate (APT) as the precursor. An as-anodized TiO2 nanotube foil was dipped into an APT aqueous solution with different molarities (i.e., 0.1, 0.3, 0.5, 1, and 5 mM) for 1 h. The samples were rinsed with deionized water and then dried with a stream of N2. Subsequently, the samples were thermal-annealed at 400 °C in an argon atmosphere for 4 h, which decomposed the APT into tungsten trioxide (WO3).
In summary, WO3-TiO2 nanotubes have been prepared using ammonium paratungstate for PEC water splitting, a maximum photocurrent of 2.1 mA/cm2 with a photoconversion efficiency of 5.1% was obtained. The WO3–TiO2 nanotubes dipped in 0.3 mM APT aqueous solution exhibited better photoelectrochemical water-splitting performance under visible illumination. In this case, the optimum WO3 content acted as an electron acceptor, which was beneficial for the effective separation of the photo-induced charge carriers.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com