Boron Doped-Tungsten Trioxide Films for PEC Water Splitting
- Details
- Category: Tungsten Information
- Published on Friday, 20 August 2021 02:04
In photoelectrochemical (PEC) water splitting, hydrogen is produced from water using sunlight and specialized semiconductors called photoelectrochemical materials, which use light energy to directly dissociate water molecules into hydrogen and oxygen. Hydrogen is the most promising clean energy source with very high gravimetric energy density and environmental friendliness.
Most of the recent research on H2 production via photoelectrochemical (PEC) water splitting has focused on the use of n-type semiconductor metal oxide materials employed as photoanodes. However, PEC devices suffer from low stability and efficiency, preventing them to penetrate the market. Thus, it is necessary to enhance the photoelectrochemical performance of PEC water splitting.

It is reported that boron had been applied as a dopant to nanostructured WO3 films by one-step, sol–gel method. Boron doped-tungsten trioxide films (B-WO3) has been synthesized for photoelectrochemical (PEC) water splitting, the PEC performance improves greatly by 25% compared to undoped WO3 films. The B-WO3 photoanodes has been extensively enhanced by about 25% water oxidation photocurrents which attain 2.15 mA/cm2 at 1.23 V versus reversible hydrogen electrode (RHE), under standard solar conditions (AM 1.5). The synthesis process of B-WO3 is as below:
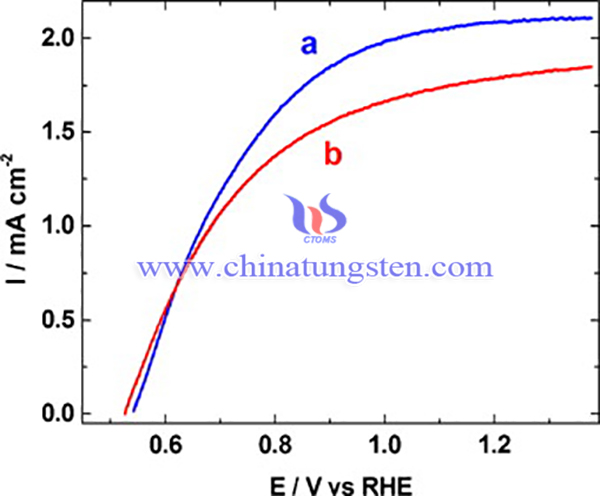
Firstly, nanoscale WO3 films with thickness of approximately 1.2 μm were formed using a sol–gel method. Then, the precursor made up of (poly)tungstic acid, freshly prepared by elution of a solution of Na2WO4 through the cation exchange column, and of an organic complexing agent (acting also as porogen), low molecular weight polyethylene glycol (PEG) 300. The WO3/PEG ratio was 0.5 w/w. Boric acid (H3BO3) was a source of boron added to the precursor to obtain 10 at.%, respectively, 20 at.% B/W ratios. Fluorine-doped SnO2 (FTO) conductive glass (Pilkington Glass, resistance 8–10 Ω/square) was used as a substrate. The FTO samples were first cleaned with acetone and then sonicated in deionized water for several hours. Some drops of the colloidal precursor were spread on the FTO substrate using doctor-blade technique, dried in air at 100 °C and, subsequently, samples were annealed in flowing oxygen at 550 °C for 30 min. The thickness of single WO3 layers was 0.35–0.45 μm; thicker films were formed by consecutive applications of the precursor, each followed by the annealing.
In summary, Boron doped-tungsten trioxide films has been synthesized for photoelectrochemical (PEC) water splitting, the PEC performance improves greatly by 25% compared to undoped WO3 films. The photoelectrochemical (PEC) studies performed under simulated solar AM 1.5 illumination showed significantly enhanced water oxidation photocurrents for B-WO3 photoanodes, by about 25% higher than those for the undoped WO3 films of similar thickness.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com