Recovery of Tantalum By Crystallization of Ammonium Paratungstate (APT) from Mother Liquor
- Details
- Category: Tungsten Information
- Published on Friday, 12 June 2020 05:52
It is found that tantalum (Ta) and niobium (Nb) in concentrate of wolframite ((Fe,Mn)WO4) has been wasted: some of it goes to the insoluble residue after ammonium-leaching of the tungstic acid slurry; most of it goes to the solution of ammonium tungstate when (Fe,Mn)WO4 concentrate was digested by hydrochloric acid and the slurry of tungstic acid obtained was leached in ammonia. When the ammonium paratungstate (APT) was crystallized from the solution of ammonium tungstate, Ta and No were almost completely left in the mother liquor and their content reached up to 16 g/litre.
At the steps of (Fe,Mn)WO4 concentrate digestion, there is a large quantity of tungstic acid gathered by tantalic and niobic acid. Therefore, it was supposed that during the am- monium leaching of tungstic acid slurry, the solubility of tantalic acid and niobic acid was due to the existence of tungstic acid. It was supposed that tantalum and niobium existed in the form of heteropoly-compounds in the mother liquor after crystallization of APT.
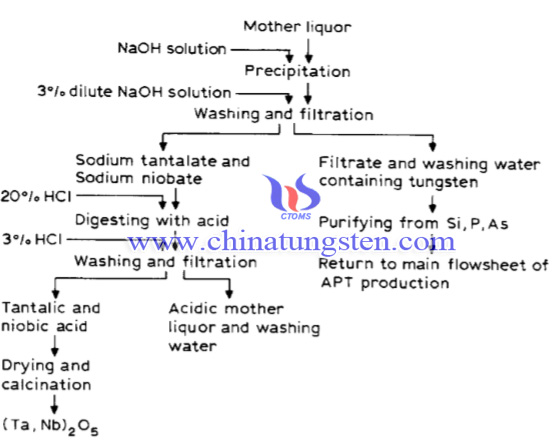
To avoid the loss of Ta and Nb, a recovery process from the mother liquor through crystallization of APT has been created. Here are the recovery steps:
The reaction was conducted in a stainless steel reactor with a volume of 10 m3. Mother liquor of 6-7 m3 was heated until 100°C with heat steam. Next, add concentrated caustic soda solution to the mother liquor with a flow of compressed air. When no more ammonia was set free from the solution,
The solution was filtered. The precipitate obtained was coloured brown, but the filtrate was colourless or yellowish. In order to purify the tantalum-niobium product, the precipitate containing tantalum and niobium was washed with 3% NaOH solution and then further digested with 20 % commercial hydrochloric acid. The crude tantalic and niobic acid obtained was filtered and washed with 3 % technical hydrochloric acid several times, then ignited to the oxide in a muffle furnace..
In conclusion, it was considered that Ta and Nb existed in the form of heteropoly-compounds in the mother liquor after crystallization of ammonium paratungstate.By adding caustic soda solution to the mother liquor while agitating at a temperature of 100°C, tantalum and niobium are precipitated as sodium tantalate and niobate. The precipitate is digested with 20 % hydrochloric acid and washed with 3 % hydrochloric acid. After drying and calcining, the raw tantalum and niobium oxide is then obtained.
The content of crude precipitation of Ta and No recovered ranged from 57 % to 86 %. This material can be used as raw material for the preparation of pure Ta2O and Nb2O5. When the content of NaOH in the solution was 35-40 g/litre, the recovery of tantalum and niobium was over 96%. When the alkalinity of the solution was lower, the quantity of NaCl added was found to have a significant influence on the recovery of tantalum and niobium.
- APT Manufacturer & Supplier, Chinatungsten Online: ammonium-paratungstate.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com