Low-dimensional Nanostructured Tungsten Trioxide
- Details
- Category: Tungsten Information
- Published on Tuesday, 12 February 2019 22:50
The crystal structure of tungsten trioxide belongs to ReO3 type and its band gap is about 2.8eV. Tungsten trioxide has unique physical and chemical properties. It has wide application prospects in gas sensing, electrochemistry, electrochromic, photocatalysis and other fields.
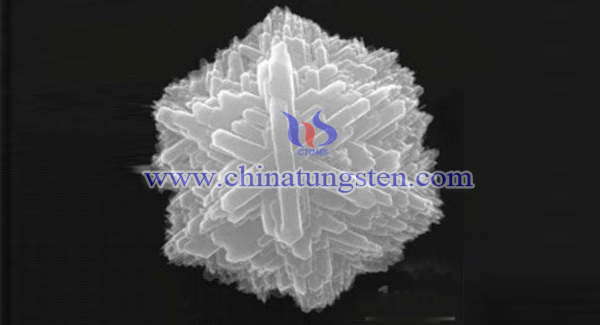
Two-dimensional tungsten oxide nanoribbons with different morphologies and sizes can be obtained by solvothermal method using tungsten source, alcohol solvent and hydrogen peroxide as raw materials by changing precursor solvents, reaction time and reaction temperature. The nanoribbons are further self-assembled to form three-dimensional tungsten oxide micro-nanostars with controllable size and uniform morphology, which include the following steps:
1.Alcohol solvents and tungsten sources are added to the reaction vessel; the alcohol solvents mentioned above are ethanol; tungsten sources are tungsten powder, and the mass ratio of tungsten powder to ethanol is 1:80-1:200.
2.Slowly add hydrogen peroxide to the reaction vessel and stir until the tungsten source dissolves completely to form a uniform and stable colloid.
3.Transfer the colloidal solution in the reaction vessel to the reactor to raise the temperature to 140-220 ℃, and keep it for 60-600 minutes.
4.Remove the supernatant from the reactor, wash and centrifuge the bottom part of the reactor repeatedly with organic solvents to obtain precipitation, which is a micro-nanostar formed by self-assembly of tungsten trioxide nanoribbons.
The products were characterized and analyzed. The results showed that the orthorhombic tungsten trioxide nanostars with regular morphology and about 3 micron diameter were obtained according to the process. SEM showed that the nanostars were self-assembled from tungsten trioxide nanoribbons. The nanostars had excellent crystallinity, uniform size and shape, adjustable size, high specific surface area, and had high specific surface area in the field of electrochemistry and sensing. It has great application prospects.
- Tungsten Oxide Manufacturer & Supplier, Chinatungsten Online: www.tungsten-oxide.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com