How Rare Earth Elements Make Modern Technology Possible
- Details
- Category: Tungsten's News
- Published on Saturday, 27 May 2023 19:38
In Frank Herbert's space opera Dune, a precious natural substance called "spice mixture" gives people the ability to navigate the vast universe to build an interstellar civilization. In real life on Earth, a group of natural metals known as rare earth elements make our modern technology possible. The demand for these key components in virtually all modern electronics is skyrocketing.

Rare earths meet thousands of different needs - cerium, for example, is used as a catalyst in oil refining, while gadolinium traps neutrons in nuclear reactors. But the most outstanding abilities of these elements are their luminescence and magnetism.
We rely on rare earths to color our smartphone screens, fluoresce to indicate the authenticity of euro banknotes, and relay signals across the ocean floor via fiber optic cables. They're also necessary to make some of the world's strongest and most reliable magnets. They produce sound waves in your headphones, boost digital information in space and alter the trajectory of heat-seeking missiles. Rare earths are also driving green technologies, such as wind energy and electric cars, and may even yield new components for quantum computers. Stephen Boyd, a synthetic chemist and independent consultant, says, "The list goes on and on. They're everywhere."
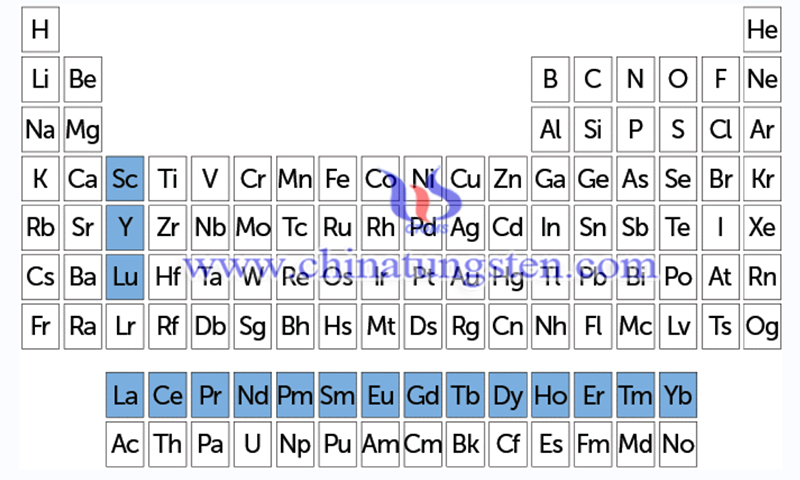
Rare earths are all 14 elements in the lanthanide family - lutetium and between lanthanum and ytterbium, spanning one row of the periodic table - plus scandium and yttrium, which tend to occur in the same deposits and have chemical properties similar to those of the lanthanides. These gray to silver-colored metals are usually malleable and have high melting and boiling points.
Their secret power lies in their electrons. All atoms have a nucleus surrounded by electrons that reside in regions called orbitals. The electrons in the orbitals farthest from the nucleus are valence electrons, which are involved in chemical reactions and form bonds with other atoms.
Most lanthanides have another important group of electrons, called "f-electrons," that reside in the golden zone near the valence electrons but slightly closer to the nucleus. Ana de Bettencourt-Dias, an inorganic chemist at the University of Nevada, Reno, says, "It's these f-electrons that lead to the magnetic and luminescent properties of rare earth elements."
Rare earths are a group of 17 elements (marked in blue on the periodic table). A subset of the rare earth elements is known as the lanthanides (lutetium, Lu, plus a row headed by lanthanum, La), and each element contains a subshell that usually holds the f-electron, which gives these elements their magnetic and luminescent properties.
Reference: https://www.sciencenews.org/article/rare-earth-elements-properties-technology
- Rare Earth Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com