MoS2 Cathode Modified with Glutamic Acid
- Details
- Category: Tungsten's News
- Published on Monday, 28 December 2020 20:44
Jinan University modifies molybdenum disulfide (MoS2) cathode material with glutamic acid. Recently, Hongzhan Chen, a 2018 undergraduate student majoring in Applied Physics at the College of Science and Technology of Jinan University, used the method of amino acid modification to successfully prepare a MoS2 cathode material modified with glutamic acid.
The material accelerates the dehydration of the hydrated zinc ions in the zinc-ion battery by initiating the interface polarization, thereby making it easier for the zinc ions to be embedded in the positive electrode material to obtain a chemical battery with a larger specific capacity.
At present, the more mature technology of lithium-ion batteries owns the advantages such as high output voltage and long service life, and has been widely applied in mobile communication terminals, electric vehicles and other fields. However, the high cost, low safety, and environmental pollution during the preparation and recycling of lithium-ion batteries limit its further development. Therefore, the development of low-cost, high-safety, and stable operation of secondary battery technology has always attracted great attention from countries all over the world.
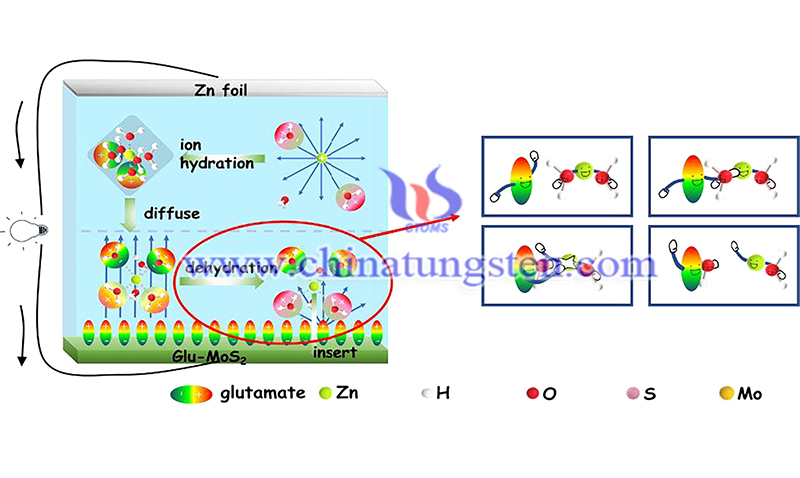
Rechargeable zinc-ion batteries the advantages of high safety, easy assembly, low cost, environmental friendliness and rich zinc resources, and are expected to become the ideal choice for the next generation of electrochemical energy storage devices. However, due to the large resistance of zinc ions intercalation into the molybdenum disulfide cathode material, the capacity of the zinc ion battery is greatly restricted.
To reduce the resistance of zinc ions inserted into the molybdenum disulfide cathode material, Chen Hongzhan used the amino acid surface modification method to modify the MoS2 cathode material, which can make it easier for zinc ions to be extracted and inserted to increase the specific energy density of the product. Moreover, the modification method does not change the structure of the modified material, which means that other materials can also be surface modified with amino acids to improve the corresponding performance.
The huge activation energy barrier during the dehydration of hydrated zinc ions of aqueous zinc-ion batteries (ZIBs) has limited their capacity and hindered their development. The researchers report a novel and efficient method for accelerating the dehydration of hydrated zinc ions through interfacial polarization triggered by glutamic acid.
Using MoS2 as a research model, the specific capacity adsorbed by glutamate increased from 72 mAh g−1 to 182 mAh g−1, which is 2.5 times the original specific capacity. Different from changing the structure of materials, surface treatment also can significantly improve performance.
In addition, there is a reversible phase conversion from the 2H phase to the 1 T phase during the charging and discharging process of Glu- MoS2, which plays an important role in optimizing the performance. The interfacial polarization caused by glutamate to reduce the activation energy of dehydration provides a new strategy to increase the capacity of ZIBs.
The research results titled "Interfacial polarization triggered by glutamate accelerates dehydration of hydrated zinc ions for zinc-ion batteries" has been published in internationally renowned journal.
| Molybdenum Supplier: Chinatungsten Online www.molybdenum.com.cn | Tel.: 86 592 5129696; Fax: 86 592 5129797;Email:sales@chinatungsten.com |
| Tungsten News & Prices, 3G Version: http://3g.chinatungsten.com | Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn |



 sales@chinatungsten.com
sales@chinatungsten.com