Scientists Develop LLTO Anode Material for Lithium Batteries
- Details
- Category: Tungsten's News
- Published on Thursday, 27 August 2020 21:36
Researchers from the Karlsruhe Institute of Technology (KIT) in Germany and Jilin University in Changchun, China have investigated a highly promising anode material for future high-performance lithium batteries: lithium lanthanum titanate with a perovskite crystal structure (LLTO). As the team reported in the journal Nature Communications, LLTO can increase the energy density, power density, charging rate, safety, and cycle life of batteries without requiring a decrease of the particle size from microscale to nanoscale.
The demand for electric vehicles is growing, and at the same time, the demand for smart grids that ensure a sustainable energy supply is also growing. These and other mobile and stationary technologies require suitable batteries. Store as much energy as possible with the smallest weight in the smallest possible space-lithium ion batteries (LIB) can still best meet this requirement. The research aims to improve the energy density, power density, safety, and cycle life of these batteries. The electrode material is crucial.
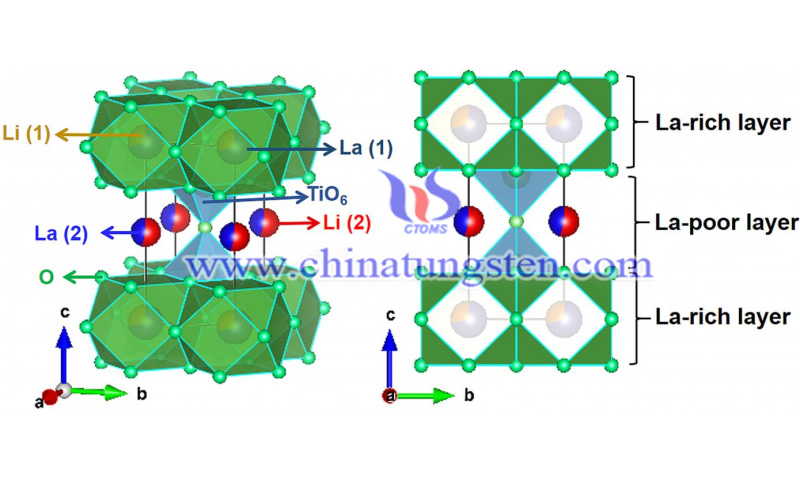
The anode of lithium batteries consists of a current collector and an active material applied to it, which stores energy in the form of chemical bonds. In most cases, graphite is used as the active material. However, negative electrodes made of graphite have a low charging rate. Moreover, they are associated with safety issues. Among the alternative active materials, lithium titanate oxide (LTO) has already been commercialized. The negative electrode with LTO has a higher charge rate and is considered safer than electrodes made of graphite. The disadvantage is that lithium-ion batteries with lithium titanate oxide tend to have lower energy density.
The team around Professor Helmut Ehrenberg, head of the Institute of Applied Materials for KIT Energy Storage Systems (IAM-ESS), has now studied another highly promising anode material: lithium lanthanum titanate with a perovskite crystal structure. According to research conducted in collaboration with scientists from Jilin University (China) and other research institutions in China and Singapore, LLTO anodes have a lower electrode potential compared to commercialized LTO anodes, which allows for a higher cell voltage and a higher capacity.
"Cell voltage and storage capacity ultimately determine the energy density of a battery", Helmut Ehrenberg explained: "In the future, LLTO anodes might be used to build particularly safe high-performance cells with long cycle life. " The research contributes to the work of the research platform for electrochemical storage, CELEST (Center for Electrochemical Energy Storage Ulm & Karlsruhe), one of the largest battery research platforms worldwide, which also includes the POLiS excellence cluster.
In addition to energy density, power density, safety, and cycle life, the charging rate is another decisive factor in determining whether a battery is suitable for demanding applications. In principle, the maximum discharge current and the minimum charging time depend on the ion and electron transport both within the solid body and at the interfaces between the electrode and electrolyte materials. In order to increase the charging rate, a common practice is to reduce the particle size of the electrode material from micro to nano scale.
The study showed that even particles of a few micrometers in size in LLTOs of the lithium batteries with a perovskite structure feature a higher power density and a better charging rate than LTO nanoparticles. The research team attributes this to the so-called lithium lanthanum titanate with a perovskite crystal structure pseudocapacitance: Not only are individual electrons attached to this anode material, but also charged ions, which are bound by weak forces and can reversibly transfer charges to the anode. "Because of the larger particles, LLTO enables a simpler and more cost-effective electrode manufacturing process," Ehrenberg explained.
- Tungsten Manufacturer & Supplier, Chinatungsten Online: www.chinatungsten.com
- Tungsten News & Prices of China Tungsten Industry Association: www.ctia.com.cn
- Molybdenum News & Price: news.molybdenum.com.cn
- Tel.: 86 592 5129696; Fax: 86 592 5129797; Email: sales@chinatungsten.com



 sales@chinatungsten.com
sales@chinatungsten.com