Pyrochlore Type Oxide Reaction Mechanism
- Details
- Category: Tungsten Information
- Published on Tuesday, 07 April 2015 15:45
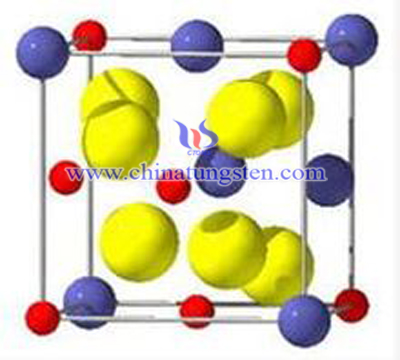
Pyrochlore type oxide, tungsten oxide (often referred to as H2W2O7 or WO • -0.5H2O) to WO6 distorted octahedral structural motifs, by a rigid skeleton top corner WO6 construct a layered structure with the bore having a six-membered ring, in layers formed between road. Which has an n-type semiconductor properties and microwave absorption properties, as a new industrial materials, it photochromic, electrochromic, gas discoloration caused by aspects, catalysis have a very important significance. In addition, the tungsten oxide pyrochlore type after the firing energy directly into tungsten trioxide, tungsten trioxide can be prepared as intermediates.
Currently preparation of tungsten oxide pyrochlore type used mainly soft chemical synthesis method and hydrothermal synthesis. Hydrothermal synthesis method is widely applied in the field of inorganic materials synthesis, the advantage of easy-to-control hydrothermal conditions, the resulting product of high purity, good dispersion, size and easy to control. Currently Hydrothermal Synthesis of tungsten oxide pyrochlore type usually sodium tungstate solution for raw materials, hydrothermal reaction after acidification some time to prepare. According to the findings of the different researchers, sodium tungstate solution pH in the range of 2.0 to 8.9 can hydrothermal synthesis of tungsten oxide pyrochlore type, but its synthesis mechanism is not clear, no direct evidence to determine the reaction precursor, study needs further. The presence of an acidic environment with a variety of tungsten ions and more heteropolyacid ion, and the transformation of the structure of the situation is complex and varied, this article focuses on the basic system of tungsten oxide pyrochlore-type hydrothermal synthesis mechanism.
Pure sodium tungstate solution for the reactants, hydrochloric acid as an additive, in an alkaline environment pyrochlore type hydrothermal synthesis of tungsten oxide. Use FT-IR analysis of the infrared spectra of the test method change under different rules and acidification of the reaction solution Na2WO4 different time period, to analyze WO2-4, W7O6-24, H2W12O10-42 and W2O2-7 whether to prepare tungsten oxide pyrochlore-type reaction precursors, ruled out the use of exclusion H2W12O10-42, W2O2-7 possibility before the reaction the body as that of the precursor should WO2-4 and W7O6-24, and assisted by thermodynamic calculations prove.
Tungsten Powder Manufacturer & Supplier: Chinatungsten Online - http://www.tungsten-powder.com
Tel.: 86 592 5129696; Fax: 86 592 5129797
Email: sales@chinatungsten.com
Tungsten & Molybdenum Information Bank: http://i.chinatungsten.com
Tungsten News & Tungsten Prices, 3G Version: http://3g.chinatungsten.com
Molybdenum News & Molybdenum Price: http://news.molybdenum.com.cn



 sales@chinatungsten.com
sales@chinatungsten.com